The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon
- PMID: 18337574
- PMCID: PMC2346769
- DOI: 10.1128/JVI.02127-07
The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon
Abstract
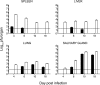
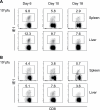
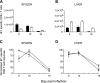
Both innate and adaptive immune responses participate in the control of murine cytomegalovirus (mCMV) infection. In some mouse strains, like BALB/c, the control of infection relies on the activities of CD8(+) T cells. mCMV-specific CD8(+) T-cell responses are unusual in that, even after mCMV has been controlled in the periphery, the numbers of circulating virus-specific CD8(+) T cells remain high compared to those observed in other viral infections. To better understand the generation and maintenance of mCMV-specific CD8(+) T-cell responses, we evaluated how antigen load and effector molecules, such as perforin (Prf) and gamma interferon (IFN-gamma), influence these responses during acute infection in vivo. Viral burden affected the magnitude, but not the early kinetics, of antigen-specific CD8(+) T-cell responses. Similarly, the magnitude of virus-specific CD8(+) T-cell expansion was affected by Prf and IFN-gamma, but contraction of antigen-specific responses occurred normally in both Prf- and IFN-gamma-deficient mice. These data indicate that control of mCMV-specific CD8(+) T-cell expansion and contraction is more complex than anticipated and, despite the role of Prf or IFN-gamma in controlling viral replication, a full program of T-cell expansion and contraction can occur in their absence.
Figures


References
-
- Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 21077-1084. - PubMed
-
- Badovinac, V. P., and J. T. Harty. 2006. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 21167-80. - PubMed
-
- Badovinac, V. P., B. B. Porter, and J. T. Harty. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5809-817. - PubMed
-
- Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3619-626. - PubMed
-
- Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 2901354-1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

