Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose
- PMID: 18337601
- PMCID: PMC2361129
- DOI: 10.1056/NEJMoa074943
Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose
Abstract
Background: Cetuximab, a chimeric mouse-human IgG1 monoclonal antibody against the epidermal growth factor receptor, is approved for use in colorectal cancer and squamous-cell carcinoma of the head and neck. A high prevalence of hypersensitivity reactions to cetuximab has been reported in some areas of the United States.
Methods: We analyzed serum samples from four groups of subjects for IgE antibodies against cetuximab: pretreatment samples from 76 case subjects who had been treated with cetuximab at multiple centers, predominantly in Tennessee, Arkansas, and North Carolina; samples from 72 control subjects in Tennessee; samples from 49 control subjects with cancer in northern California; and samples from 341 female control subjects in Boston.
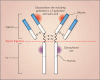
Results: Among 76 cetuximab-treated subjects, 25 had a hypersensitivity reaction to the drug. IgE antibodies against cetuximab were found in pretreatment samples from 17 of these subjects; only 1 of 51 subjects who did not have a hypersensitivity reaction had such antibodies (P<0.001). IgE antibodies against cetuximab were found in 15 of 72 samples (20.8%) from control subjects in Tennessee, in 3 of 49 samples (6.1%) from northern California, and in 2 of 341 samples (0.6%) from Boston. The IgE antibodies were shown to be specific for an oligosaccharide, galactose-alpha-1,3-galactose, which is present on the Fab portion of the cetuximab heavy chain.
Conclusions: In most subjects who had a hypersensitivity reaction to cetuximab, IgE antibodies against cetuximab were present in serum before therapy. The antibodies were specific for galactose-alpha-1,3-galactose.
Copyright 2008 Massachusetts Medical Society.
Figures

Comment in
-
Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose.N Engl J Med. 2008 Jun 19;358(25):2735; author reply 2735-6. doi: 10.1056/NEJMc080834. N Engl J Med. 2008. PMID: 18565869 No abstract available.
References
-
- Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. - PubMed
-
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. - PubMed
-
- Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role in IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–65. - PubMed
-
- Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–24. - PubMed
-
- Cook-Bruns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology. 2001;61(Suppl 2):58–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DE-017982/DE/NIDCR NIH HHS/United States
- P50-CA-95103/CA/NCI NIH HHS/United States
- AI-20565/AI/NIAID NIH HHS/United States
- R01-CA-118582/CA/NCI NIH HHS/United States
- R01 DE017982/DE/NIDCR NIH HHS/United States
- AI-AADCRC-U19-070364/PHS HHS/United States
- R37 AI020565/AI/NIAID NIH HHS/United States
- R29 AI035796/AI/NIAID NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01 AI020565/AI/NIAID NIH HHS/United States
- R01 AI035796/AI/NIAID NIH HHS/United States
- R01 CA118582/CA/NCI NIH HHS/United States
- AI/EHS-35796/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
