Antitumor activity of noscapine in human non-small cell lung cancer xenograft model
- PMID: 18338172
- PMCID: PMC7799395
- DOI: 10.1007/s00280-008-0720-z
Antitumor activity of noscapine in human non-small cell lung cancer xenograft model
Abstract
Purpose: An antitussive plant alkaloid, Noscapine HCl (Nos) displays anticancer activity and has a safe pharmacological profile in humans. The current study was aimed to investigate the in vitro and in vivo anti tumor activity of Nos to determine possible mechanisms of anti tumor activity for treatment of non-small cell lung cancer (NSCLC).
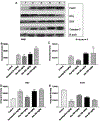
Methods: In vitro cytotoxicity of Nos was studied in H460 cells treated with different doses of Nos (10-160 microM) for 72 h and cell viability was determined using crystal violet assay. Apoptosis in H460 cells was evaluated by TUNEL assay after treatment of cells for 72 h with 30 and 40 microM doses of Nos. For in vivo studies, female athymic Nu/nu mice were xenografted with H460 tumors and on day 4 onwards Nos was administered orally at dose of 300, 450 and 550 mg/kg/day for 24 days. As a control, xenografted tumors were separately treated with Docetaxel (10 mg/kg i.v. bolus on day 5, 11, 17, 23). The tumor volumes were measured every five days. Expression of PARP, Bcl(2, )Bax, and caspase-3 families of proteins was measured by Western Blotting (WB), while TUNEL and Immunohistochemical methods were utilized to determine DNA fragmentation and cleaved caspase-3 levels respectively.
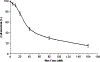
Results: Nos inhibited growth of H460 cells with the IC50 values of 34.7 +/- 2.5 microM. Nos at 30 and 40 microM doses caused apoptosis as evidenced by nuclear condensation in treated H460 cells. Nos caused 49, 65 and 86% reduction in the xenografted tumor volumes at a dose of 300 (P < 0.05), 450 (P < 0.01), 550 mg/kg/day (P < 0.01), respectively, when compared to controls. Nos-dependent suppression of xenografted tumor growth involved up regulation of PARP, Bax, caspase-3 and repression of Bcl(2) expression. An increase in Bax/Bcl(2) ratio suggests involvement of a mitochondrial mediated apoptotic processes. Our studies revealed a non significant (P > 0.05) increase in Bax/Bcl(2) ratio with Nos at a dose of 300 mg/kg/day, while a significant (P < 0.001) increase in Bax/Bcl(2) ratio was observed with Nos doses of 450 and 550 mg/kg/day. Further, Nos caused elevated apoptosis in tumor xenografts as evidenced by enhanced expression of caspase-3 and positive TUNEL staining in regressed tumor tissues, thus suggesting induction of apoptosis by mitochondrial pathway.
Conclusion: Our studies suggest that potent antitumor activity of Nos against NSCLC cells. Oral administration of Nos showed significant reduction in tumor volume in human non-small cell lung tumor xenograft in nude mice in a dose dependant manner. Thus, Nos is a promising novel chemotherapeutic agent for the treatment of human lung cancer.
Figures




Similar articles
-
Enhanced anticancer activity of gemcitabine in combination with noscapine via antiangiogenic and apoptotic pathway against non-small cell lung cancer.PLoS One. 2011;6(11):e27394. doi: 10.1371/journal.pone.0027394. Epub 2011 Nov 15. PLoS One. 2011. PMID: 22102891 Free PMC article.
-
Anticancer activity of Noscapine, an opioid alkaloid in combination with Cisplatin in human non-small cell lung cancer.Lung Cancer. 2011 Mar;71(3):271-82. doi: 10.1016/j.lungcan.2010.06.002. Epub 2010 Jul 31. Lung Cancer. 2011. PMID: 20674069 Free PMC article.
-
Chloroquine potentiates the anti-cancer effect of lidamycin on non-small cell lung cancer cells in vitro.Acta Pharmacol Sin. 2014 May;35(5):645-52. doi: 10.1038/aps.2014.3. Epub 2014 Apr 14. Acta Pharmacol Sin. 2014. PMID: 24727941 Free PMC article.
-
Noscapine and Apoptosis in Breast and Other Cancers.Int J Mol Sci. 2024 Mar 21;25(6):3536. doi: 10.3390/ijms25063536. Int J Mol Sci. 2024. PMID: 38542508 Free PMC article. Review.
-
Fantastic Frogs and Where to Use Them: Unveiling the Hidden Cinobufagin's Promise in Combating Lung Cancer Development and Progression Through a Systematic Review of Preclinical Evidence.Cancers (Basel). 2024 Nov 7;16(22):3758. doi: 10.3390/cancers16223758. Cancers (Basel). 2024. PMID: 39594713 Free PMC article. Review.
Cited by
-
Synergistic Anticancer Effect of Paclitaxel and Noscapine on Human Prostate Cancer Cell Lines.Iran J Pharm Res. 2017 Fall;16(4):1432-1442. Iran J Pharm Res. 2017. PMID: 29552052 Free PMC article.
-
Formulation, Pharmacokinetic, and Efficacy Studies of Mannosylated Self-Emulsifying Solid Dispersions of Noscapine.PLoS One. 2016 Jan 12;11(1):e0146804. doi: 10.1371/journal.pone.0146804. eCollection 2016. PLoS One. 2016. PMID: 26757437 Free PMC article.
-
Nuclear and cytoplasmic p53 suppress cell invasion by inhibiting respiratory complex-I activity via Bcl-2 family proteins.Oncotarget. 2014 Sep 30;5(18):8452-65. doi: 10.18632/oncotarget.2320. Oncotarget. 2014. PMID: 25115399 Free PMC article.
-
Metabolic map and bioactivation of the anti-tumour drug noscapine.Br J Pharmacol. 2012 Nov;167(6):1271-86. doi: 10.1111/j.1476-5381.2012.02067.x. Br J Pharmacol. 2012. PMID: 22671862 Free PMC article.
-
Opium Consumption and the Incidence of Cancer: Does Opium Account as an Emerging Risk Factor for Gastrointestinal Cancer?J Gastrointest Cancer. 2018 Jun;49(2):172-180. doi: 10.1007/s12029-017-0050-7. J Gastrointest Cancer. 2018. PMID: 29362985
References
-
- Wakelee H, Belani CP (2005) Optimizing First-Line treatment options for patients with advanced NSCLC. Oncologist 10:1–10 - PubMed
-
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR (2004) American Society of Clinical Oncology Treatment of Unresectable Non-Small-Cell Lung Cancer Guideline: Update 2003. J Clin Oncol 22(2):330–353 - PubMed
-
- The International Adjuvant Lung Cancer Trial Collaborative Group (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med 350:351–360 - PubMed
-
- Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, Fry W, Bethune D, Ayoub J, Ding K, Seymour L, Graham B, Tsao MS, Gandara D, Kesler K, Demmy T, Shepherd F (2005) Vinorelbine plus cisplatin vs. observation in resected non-small cell lung cancer. N Engl J Med 352:2589–2597 - PubMed
-
- Strauss GM, Herndon J, Maddaus MA, Johnstone DW, Johnson EA, Watson DM, Sugarbaker DJ, Schilsky RL, Green MR (2004) Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in stage IB non-small cell lung cancer: report of Cancer and Leukemia Group B protocol 9633. J Clin Oncol, 22(14S):621s, Abstract #7019
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

