Wnt/beta-catenin signaling: new (and old) players and new insights
- PMID: 18339531
- PMCID: PMC2390924
- DOI: 10.1016/j.ceb.2008.01.009
Wnt/beta-catenin signaling: new (and old) players and new insights
Abstract
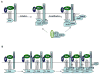
Wnt/beta-catenin signaling has central roles in embryogenesis and human diseases including cancer. A central scheme of the Wnt pathway is to stabilize the transcription coactivator beta-catenin by preventing its phosphorylation-dependent degradation. Significant progress has been made toward the understanding of this crucial regulatory pathway, including the protein complex that promotes beta-catenin phosphorylation-degradation, and the mechanism by which the extracellular Wnt ligand engages cell surface receptors to inhibit beta-catenin phosphorylation-degradation. Here we review some recent discoveries in these two areas, and highlight some crucial questions that remain to be resolved.
Figures

References
-
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. - PubMed
-
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. - PubMed
-
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

