Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease
- PMID: 18339640
- PMCID: PMC2408940
- DOI: 10.1093/brain/awn016
Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease
Abstract
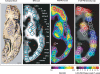
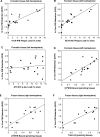
The positron emission tomography (PET) radiotracer Pittsburgh Compound-B (PiB) binds with high affinity to beta-pleated sheet aggregates of the amyloid-beta (Abeta) peptide in vitro. The in vivo retention of PiB in brains of people with Alzheimer's disease shows a regional distribution that is very similar to distribution of Abeta deposits observed post-mortem. However, the basis for regional variations in PiB binding in vivo, and the extent to which it binds to different types of Abeta-containing plaques and tau-containing neurofibrillary tangles (NFT), has not been thoroughly investigated. The present study examined 28 clinically diagnosed and autopsy-confirmed Alzheimer's disease subjects, including one Alzheimer's disease subject who had undergone PiB-PET imaging 10 months prior to death, to evaluate region- and substrate-specific binding of the highly fluorescent PiB derivative 6-CN-PiB. These data were then correlated with region-matched Abeta plaque load and peptide levels, [(3)H]PiB binding in vitro, and in vivo PET retention levels. We found that in Alzheimer's disease brain tissue sections, the preponderance of 6-CN-PiB binding is in plaques immunoreactive to either Abeta42 or Abeta40, and to vascular Abeta deposits. 6-CN-PiB labelling was most robust in compact/cored plaques in the prefrontal and temporal cortices. While diffuse plaques, including those in caudate nucleus and presubiculum, were less prominently labelled, amorphous Abeta plaques in the cerebellum were not detectable with 6-CN-PiB. Only a small subset of NFT were 6-CN-PiB positive; these resembled extracellular 'ghost' NFT. In Alzheimer's disease brain tissue homogenates, there was a direct correlation between [(3)H]PiB binding and insoluble Abeta peptide levels. In the Alzheimer's disease subject who underwent PiB-PET prior to death, in vivo PiB retention levels correlated directly with region-matched post-mortem measures of [(3)H]PiB binding, insoluble Abeta peptide levels, 6-CN-PiB- and Abeta plaque load, but not with measures of NFT. These results demonstrate, in a typical Alzheimer's disease brain, that PiB binding is highly selective for insoluble (fibrillar) Abeta deposits, and not for neurofibrillary pathology. The strong direct correlation of in vivo PiB retention with region-matched quantitative analyses of Abeta plaques in the same subject supports the validity of PiB-PET imaging as a method for in vivo evaluation of Abeta plaque burden.
Figures




References
-
- Consensus report of the Working Group on “Molecular and Biochemical Markers of Alzheimer's Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol Aging. 1998;19:109–16. - PubMed
-
- Bacskai BJ, Frosch MP, Freeman SH, Raymond SB, Augustinack JC, Johnson KA, et al. Molecular imaging with PiB confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–4. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropath. 1991a;82:239–59. - PubMed
-
- Braak H, Braak E. Neuropathological staging of Alzheimer's disease. Acta Neuropath. 1991b;82:239–59. - PubMed
-
- DeKosky ST, Abrahamson EE, Ciallella JR, Paljug WR, Wisniewski SR, Clark RSB, et al. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch Neurol. 2007;64:541–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG14449/AG/NIA NIH HHS/United States
- K01 MH001976/MH/NIMH NIH HHS/United States
- R01 AG018402/AG/NIA NIH HHS/United States
- R01 MH070729/MH/NIMH NIH HHS/United States
- R37 AG025516/AG/NIA NIH HHS/United States
- AG025516/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 AG05133/AG/NIA NIH HHS/United States
- RF1 AG025516/AG/NIA NIH HHS/United States
- R01 AG020226/AG/NIA NIH HHS/United States
- K02 AG001039/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
- R01 AG025516/AG/NIA NIH HHS/United States
- AG05133/AG/NIA NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

