Molecular characterization of V59E NIS, a Na+/I- symporter mutant that causes congenital I- transport defect
- PMID: 18339708
- PMCID: PMC2408800
- DOI: 10.1210/en.2008-0027
Molecular characterization of V59E NIS, a Na+/I- symporter mutant that causes congenital I- transport defect
Abstract
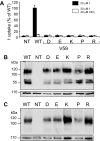
I(-) is actively transported into thyrocytes via the Na+/I(-) symporter (NIS), a key glycoprotein located on the basolateral plasma membrane. The cDNA encoding rat NIS was identified in our laboratory, where an extensive structure/function characterization of NIS is being conducted. Several NIS mutants have been identified as causes of congenital I(-) transport defect (ITD), including V59E NIS. ITD is characterized by low thyroid I(-) uptake, low saliva/plasma I(-) ratio, hypothyroidism, and goiter and may cause mental retardation if untreated. Studies of other ITD-causing NIS mutants have revealed valuable information regarding NIS structure/function. V59E NIS was reported to exhibit as much as 30% of the activity of wild-type NIS. However, this observation was at variance with the patients' phenotype of total lack of activity. We have thoroughly characterized V59E NIS and studied several amino acid substitutions at position 59. We demonstrated that, in contrast to the previous report, V59E NIS is inactive, although it is properly targeted to the plasma membrane. Glu and all other charged amino acids or Pro at position 59 also yielded nonfunctional NIS proteins. However, I(-) uptake was rescued to different degrees by the other substitutions. Although the Km values for Na+ and I(-) were not altered in these active mutants, we found that the structural requirement for NIS function at position 59 is a neutral, helix-promoting amino acid. This result suggests that the region that contains V59 may be involved in intramembrane helix-helix interactions during the transport cycle without being in direct contact with the substrates.
Figures





References
-
- Carrasco N 1993 Iodide transport in the thyroid gland. Biochim Biophys Acta 1154:65–82 - PubMed
-
- Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N 1997 Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem 272:27230–27238 - PubMed
-
- Dai G, Levy O, Carrasco N 1996 Cloning and characterization of the thyroid iodide transporter. Nature 379:458–460 - PubMed
-
- De la Vieja A, Dohan O, Levy O, Carrasco N 2000 Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev 80:1083–1105 - PubMed
-
- Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N 2003 The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

