A biophysically based mathematical model of unitary potential activity in interstitial cells of Cajal
- PMID: 18339738
- PMCID: PMC2426626
- DOI: 10.1529/biophysj.107.122507
A biophysically based mathematical model of unitary potential activity in interstitial cells of Cajal
Abstract
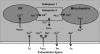
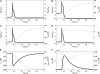
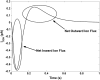
Unitary potential (UP) depolarizations are the basic intracellular events responsible for pacemaker activity in interstitial cells of Cajal (ICCs), and are generated at intracellular sites termed "pacemaker units". In this study, we present a mathematical model of the transmembrane ion flows and intracellular Ca(2+) dynamics from a single ICC pacemaker unit acting at near-resting membrane potential. This model quantitatively formalizes the framework of a novel ICC pacemaking mechanism that has recently been proposed. Model simulations produce spontaneously rhythmic UP depolarizations with an amplitude of approximately 3 mV at a frequency of 0.05 Hz. The model predicts that the main inward currents, carried by a Ca(2+)-inhibited nonselective cation conductance, are activated by depletion of sub-plasma-membrane [Ca(2+)] caused by sarcoendoplasmic reticulum calcium ATPase Ca(2+) sequestration. Furthermore, pacemaker activity predicted by our model persists under simulated voltage clamp and is independent of [IP(3)] oscillations. The model presented here provides a basis to quantitatively analyze UP depolarizations and the biophysical mechanisms underlying their production.
Figures







References
-
- Szurszewski, J. H. (1987). Electrical basis for gastrointestinal motility. In Physiology of the Gastrointestinal Tract, 2nd ed. L. R. Johnson, editor. Raven Press, New York. 383–422.
-
- Tomita, T. (1981). Electrical activity (spikes and slow waves) in gastrointestinal smooth muscle. In Smooth Muscle: An Assessment of Current Knowledge. E. Bulbring, A. F. Brading, A. W. Jones, and T. Tomita, editors. University of Texas Press, Austin, TX. 127–156.
-
- Smith, T. K., J. B. Reed, and K. M. Sanders. 1987. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am. J. Physiol. 252:C215–C224. - PubMed
-
- Huizinga, J. D., L. Thuneberg, M. Kluppel, J. Malysz, H. B. Mikkelsen, and A. Bernstein. 1995. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 373:347–349. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

