X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations
- PMID: 18339804
- PMCID: PMC2290804
- DOI: 10.1073/pnas.0712018105
X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations
Abstract
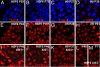
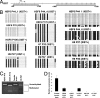
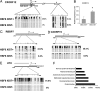
X chromosome inactivation (XCI) is an essential mechanism for dosage compensation of X-linked genes in female cells. We report that subcultures from lines of female human embryonic stem cells (hESCs) exhibit variation (0-100%) for XCI markers, including XIST RNA expression and enrichment of histone H3 lysine 27 trimethylation (H3K27me3) on the inactive X chromosome (Xi). Surprisingly, regardless of the presence or absence of XCI markers in different cultures, all female hESCs we examined (H7, H9, and HSF6 cells) exhibit a monoallelic expression pattern for a majority of X-linked genes. Our results suggest that these established female hESCs have already completed XCI during the process of derivation and/or propagation, and the XCI pattern of lines we investigated is already not random. Moreover, XIST gene expression in subsets of cultured female hESCs is unstable and subject to stable epigenetic silencing by DNA methylation. In the absence of XIST expression, approximately 12% of X-linked promoter CpG islands become hypomethylated and a portion of X-linked alleles on the Xi are reactivated. Because alterations in dosage compensation of X-linked genes could impair somatic cell function, we propose that XCI status should be routinely checked in subcultures of female hESCs, with cultures displaying XCI markers better suited for use in regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. - PubMed
-
- Adewumi O, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. - PubMed
-
- Maitra A, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. - PubMed
-
- Allegrucci C, et al. Restriction landmark genome scanning identifies culture-induced DNA methylation instability in the human embryonic stem cell epigenome. Hum Mol Genet. 2007;16:1253–1268. - PubMed
-
- Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

