Role of the Akt pathway in mRNA translation of interferon-stimulated genes
- PMID: 18339807
- PMCID: PMC2290753
- DOI: 10.1073/pnas.0710907105
Role of the Akt pathway in mRNA translation of interferon-stimulated genes
Abstract
Multiple signaling pathways are engaged by the type I and II IFN receptors, but their specific roles and possible coordination in the generation of IFN-mediated biological responses remain unknown. We provide evidence that activation of Akt kinases is required for IFN-inducible engagement of the mTOR/p70 S6 kinase pathway. Our data establish that Akt activity is essential for up-regulation of key IFN-alpha- and IFN-gamma-inducible proteins, which have important functional consequences in the induction of IFN responses. Such effects of the Akt pathway are unrelated to regulatory activities on IFN-dependent STAT phosphorylation/activation or transcriptional regulation. By contrast, they reflect regulatory activities on mRNA translation via direct control of the mTOR pathway. In studies using Akt1 and Akt2 double knockout cells, we found that the absence of Akt kinases results in dramatic reduction in IFN-induced antiviral responses, establishing a critical role of the Akt pathway in IFN signaling. Thus, activation of the Akt pathway by the IFN receptors complements the function of IFN-activated JAK-STAT pathways, by allowing mRNA translation of IFN-stimulated genes and, ultimately, the induction of the biological effects of IFNs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
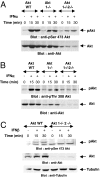

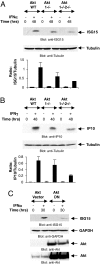
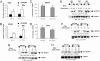
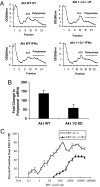
References
-
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. - PubMed
-
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1420. - PubMed
-
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. - PubMed
-
- Platanias LC, Fish EN. Signaling pathways activated by interferons. Exp Hematol. 1999;27:1583–1592. - PubMed
-
- Platanias LC. Mechanisms of type I and type II interferon-mediated signaling. Nat Rev Immunol. 2005;5:375–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

