New role of resistin in lipopolysaccharide-induced liver damage in mice
- PMID: 18339969
- PMCID: PMC2660882
- DOI: 10.1124/jpet.108.136721
New role of resistin in lipopolysaccharide-induced liver damage in mice
Abstract
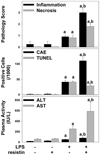
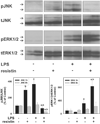
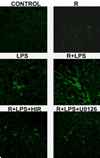
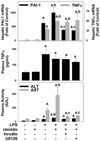
Studies in rodents suggest that the adipocytokine resistin causes insulin resistance via impairing normal insulin signaling. However, in humans, resistin may play a more important role in inflammation than in insulin resistance. Whether resistin contributes to inflammation in rodents is unclear. Therefore, the purpose of the present study was to determine the effect of resistin exposure on the basal and stimulated [lipopolysaccharide (LPS)] inflammatory response in mouse liver in vivo. Resistin alone had no major effects on hepatic expression of insulin-responsive genes, either in the presence or absence of LPS. Although it had no effect alone, resistin significantly enhanced hepatic inflammation and necrosis caused by LPS. Resistin increased expression of proinflammatory genes, e.g., plasminogen activator inhibitor (PAI)-1, and activity of mitogen-activated protein (MAP) kinase, extracellular signal-regulated kinase 1/2, caused by LPS, but had little effect on anti-inflammatory gene expression. Resistin also enhanced fibrin deposition (an index of hemostasis) caused by LPS. The increase in PAI-1 expression, fibrin deposition, and liver damage caused by LPS + resistin was almost completely prevented either by inhibiting the coagulation cascade, hirudin, or by blocking MAP kinase signaling, U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene], indicating that these pathways play a causal role in observed enhanced liver damage caused by resistin. Taken together, the augmentation of LPS-induced liver damage caused by resistin seems to involve, at least in part, up-regulation of hepatic inflammation via mechanisms most likely involving the coagulation cascade and fibrin accumulation. These data also suggest that resistin may have proinflammatory roles in mouse liver independent of its effects on insulin signaling, analogous to previous work in humans.
Figures



Similar articles
-
Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice.Hepatology. 2009 May;49(5):1545-53. doi: 10.1002/hep.22847. Hepatology. 2009. PMID: 19291788 Free PMC article.
-
Hepatoprotective effect of cryptotanshinone from Salvia miltiorrhiza in D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure.Phytomedicine. 2014 Jan 15;21(2):141-7. doi: 10.1016/j.phymed.2013.07.016. Epub 2013 Sep 4. Phytomedicine. 2014. PMID: 24011530
-
Chronological expression of PAR isoforms in acute liver injury and its amelioration by PAR2 blockade in a rat model of sepsis.Thromb Haemost. 2006 Dec;96(6):830-8. Thromb Haemost. 2006. PMID: 17139380
-
Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice.Hepatology. 2004 Jul;40(1):177-84. doi: 10.1002/hep.20282. Hepatology. 2004. PMID: 15239101
-
Protective effect of Xuebijing injection on D-galactosamine- and lipopolysaccharide-induced acute liver injury in rats through the regulation of p38 MAPK, MMP-9 and HO-1 expression by increasing TIPE2 expression.Int J Mol Med. 2016 Nov;38(5):1419-1432. doi: 10.3892/ijmm.2016.2749. Epub 2016 Sep 23. Int J Mol Med. 2016. PMID: 27666960 Free PMC article.
Cited by
-
Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice.Toxicol Appl Pharmacol. 2011 Dec 15;257(3):356-64. doi: 10.1016/j.taap.2011.09.019. Epub 2011 Sep 29. Toxicol Appl Pharmacol. 2011. PMID: 21983427 Free PMC article.
-
Vinyl chloride-induced interaction of nonalcoholic and toxicant-associated steatohepatitis: Protection by the ALDH2 activator Alda-1.Redox Biol. 2019 Jun;24:101205. doi: 10.1016/j.redox.2019.101205. Epub 2019 Apr 19. Redox Biol. 2019. PMID: 31026768 Free PMC article.
-
Transient receptor potential vanilloid 1 gene deficiency ameliorates hepatic injury in a mouse model of chronic binge alcohol-induced alcoholic liver disease.Am J Pathol. 2015 Jan;185(1):43-54. doi: 10.1016/j.ajpath.2014.09.007. Epub 2014 Oct 31. Am J Pathol. 2015. PMID: 25447051 Free PMC article.
-
Resistin deficiency in mice has no effect on pulmonary responses induced by acute ozone exposure.Am J Physiol Lung Cell Mol Physiol. 2015 Nov 15;309(10):L1174-85. doi: 10.1152/ajplung.00270.2015. Epub 2015 Sep 18. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26386120 Free PMC article.
-
Role of hepatic progenitor cells in nonalcoholic fatty liver disease development: cellular cross-talks and molecular networks.Int J Mol Sci. 2013 Oct 9;14(10):20112-30. doi: 10.3390/ijms141020112. Int J Mol Sci. 2013. PMID: 24113587 Free PMC article. Review.
References
-
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. - PubMed
-
- Bergheim I, Luyendyk JP, Steele C, Russell GK, Guo L, Roth RA, Arteel GE. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther. 2006b;316:1053–1061. - PubMed
-
- Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Gines P, et al. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042–2053. - PMC - PubMed
-
- Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

