Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling
- PMID: 18340377
- PMCID: PMC2267015
- DOI: 10.1172/JCI33467
Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling
Abstract
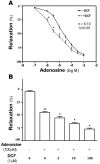
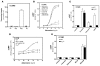
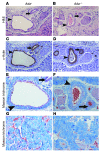
Priapism, abnormally prolonged penile erection in the absence of sexual excitation, is associated with ischemia-mediated erectile tissue damage and subsequent erectile dysfunction. It is common among males with sickle cell disease (SCD), and SCD transgenic mice are an accepted model of the disorder. Current strategies to manage priapism suffer from a poor fundamental understanding of the molecular mechanisms underlying the disorder. Here we report that mice lacking adenosine deaminase (ADA), an enzyme necessary for the breakdown of adenosine, displayed unexpected priapic activity. ADA enzyme therapy successfully corrected the priapic activity both in vivo and in vitro, suggesting that it was dependent on elevated adenosine levels. Further genetic and pharmacologic evidence demonstrated that A2B adenosine receptor-mediated (A2BR-mediated) cAMP and cGMP induction was required for elevated adenosine-induced prolonged penile erection. Finally, priapic activity in SCD transgenic mice was also caused by elevated adenosine levels and A2BR activation. Thus, we have shown that excessive adenosine accumulation in the penis contributes to priapism through increased A2BR signaling in both Ada -/- and SCD transgenic mice. These findings provide insight regarding the molecular basis of priapism and suggest that strategies to either reduce adenosine or block A2BR activation may prove beneficial in the treatment of this disorder.
Figures



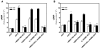

Similar articles
-
Excess adenosine A2B receptor signaling contributes to priapism through HIF-1α mediated reduction of PDE5 gene expression.FASEB J. 2014 Jun;28(6):2725-35. doi: 10.1096/fj.13-247833. Epub 2014 Mar 10. FASEB J. 2014. PMID: 24614760 Free PMC article.
-
Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling.FASEB J. 2010 Mar;24(3):740-9. doi: 10.1096/fj.09-144147. Epub 2009 Oct 26. FASEB J. 2010. PMID: 19858092 Free PMC article.
-
Adenosine signaling, priapism and novel therapies.J Sex Med. 2009 Mar;6 Suppl 3:292-301. doi: 10.1111/j.1743-6109.2008.01187.x. J Sex Med. 2009. PMID: 19267852 Review.
-
Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism.J Sex Med. 2010 Sep;7(9):3011-22. doi: 10.1111/j.1743-6109.2009.01552.x. J Sex Med. 2010. PMID: 19845544 Free PMC article.
-
Adenosine signaling: good or bad in erectile function?Arterioscler Thromb Vasc Biol. 2012 Apr;32(4):845-50. doi: 10.1161/ATVBAHA.111.226803. Arterioscler Thromb Vasc Biol. 2012. PMID: 22423035 Review.
Cited by
-
Adenosine deaminase deficiency: unanticipated benefits from the study of a rare immunodeficiency.J Immunol. 2012 Feb 1;188(3):933-5. doi: 10.4049/jimmunol.1103519. J Immunol. 2012. PMID: 22262755 Free PMC article. No abstract available.
-
Purinergic signalling in the reproductive system in health and disease.Purinergic Signal. 2014 Mar;10(1):157-87. doi: 10.1007/s11302-013-9399-7. Epub 2013 Nov 23. Purinergic Signal. 2014. PMID: 24271059 Free PMC article. Review.
-
Molecular Profile of Priapism Associated with Low Nitric Oxide Bioavailability.J Proteome Res. 2018 Mar 2;17(3):1031-1040. doi: 10.1021/acs.jproteome.7b00657. Epub 2018 Feb 12. J Proteome Res. 2018. PMID: 29394072 Free PMC article.
-
Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis.J Sex Med. 2011 Feb;8(2):419-26. doi: 10.1111/j.1743-6109.2010.02123.x. Epub 2010 Dec 8. J Sex Med. 2011. PMID: 21143412 Free PMC article.
-
Is testosterone deficiency a possible risk factor for priapism associated with sickle-cell disease?Int Urol Nephrol. 2015 Jan;47(1):47-52. doi: 10.1007/s11255-014-0864-1. Epub 2014 Nov 5. Int Urol Nephrol. 2015. PMID: 25371242 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

