Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis
- PMID: 18340379
- PMCID: PMC2267014
- DOI: 10.1172/JCI33464
Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis
Abstract
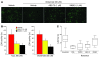
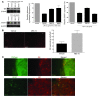
Axonal degeneration is an important determinant of progressive neurological disability in multiple sclerosis (MS). Thus, therapeutic approaches promoting neuroprotection could aid the treatment of progressive MS. Here, we used what we believe is a novel water-soluble fullerene derivative (ABS-75) attached to an NMDA receptor antagonist, which combines antioxidant and anti-excitotoxic properties, to block axonal damage and reduce disease progression in a chronic progressive EAE model. Fullerene ABS-75 treatment initiated after disease onset reduced the clinical progression of chronic EAE in NOD mice immunized with myelin-oligodendrocyte glycoprotein (MOG). Reduced disease progression in ABS-75-treated mice was associated with reduced axonal loss and demyelination in the spinal cord. Fullerene ABS-75 halted oxidative injury, CD11b+ infiltration, and CCL2 expression in the spinal cord of mice without interfering with antigen-specific T cell responses. In vitro, fullerene ABS-75 protected neurons from oxidative and glutamate-induced injury and restored glutamine synthetase and glutamate transporter expression in astrocytes under inflammatory insult. Glutamine synthetase expression was also increased in the white matter of fullerene ABS-75-treated animals. Our data demonstrate the neuroprotective effect of treatment with a fullerene compound combined with a NMDA receptor antagonist, which may be useful in the treatment of progressive MS and other neurodegenerative diseases.
Figures




References
-
- Noseworthy J.H., Lucchinetti C., Rodriguez M., Weinshenker B.G. Multiple sclerosis. N. Engl. J. Med. 2000;343:938–952. - PubMed
-
- Wujek J.R., et al. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002;61:23–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

