Absence of respiratory effects with ivabradine in patients with asthma
- PMID: 18341671
- PMCID: PMC2485243
- DOI: 10.1111/j.1365-2125.2008.03160.x
Absence of respiratory effects with ivabradine in patients with asthma
Abstract
Aim: beta-Blockers are commonly prescribed for stable angina and are recommended as initial therapy. However, beta-blockers are contraindicated in patients with obstructive airway disease because of a risk of bronchoconstriction. Ivabradine is a specific heart rate-lowering agent that acts via I(f) pacemaker channels in the sinoatrial node with no beta-adrenoreceptor activity. Ivabradine has been recently approved for the treatment of stable angina. This study assessed the effects of repeated administration of ivabradine on lung function in patients with asthma.
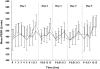
Methods: In this double-blind, placebo-controlled, crossover study, 20 subjects with asthma received either oral ivabradine 10 mg b.i.d. or placebo for 4.5 days. Forced expiratory volume in 1 s (FEV(1)) and peak expiratory flow rate (PEFR) were designated as the main outcome variable. Diary cards were used to monitor asthma symptoms on a five-point scale, rescue medication usage, and adverse events.
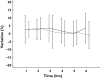
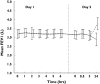
Results: There were no significant differences in mean variation of FEV(1) (ivabradine P = 0.664; placebo P = 0.652) or PEFR (ivabradine P = 0.153; placebo P = 0.356) from baseline following administration of ivabradine. There was also no significant difference in maximum percent variation in FEV(1) or PEF between treatment groups (P = 0.994; FEV(1) and P = 0.704; PEF). On a similar note, there was no significant difference in asthma symptoms or rescue medication usage reported between the two groups. Adverse events were generally mild-to-moderate in intensity and no cardiovascular or serious adverse events were recorded.
Conclusions: This study confirms that ivabradine does not affect respiratory function or symptoms in patients with asthma and therefore represents a valuable therapeutic alternative to beta-blockers for treating patients with stable angina and asthma.
Figures

References
-
- Daly CA, Clemens F, Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F Fox KM on behalf of the Euro Heart Survey Investigators. The clinical characteristics and investigations planned in patients with stable angina presenting to cardiologists in Europe: from the Euro Heart Survey of Stable Angina. Eur Heart J. 2005;26:996–1010. - PubMed
-
- Staniforth AD. Contemporary management of chronic stable angina. Drugs Aging. 2001;18:109–21. - PubMed
-
- Fihn SD, Williams SV, Daley J, Gibbons RJ. Guidelines for the management of patients with chronic stable angina: treatment. Ann Intern Med. 2001;135(8 Pt 1):616–32. - PubMed
-
- Dart RA, Gollub S, Lazar J, Nair C, Schroeder D, Woolf SH. Treatment of systemic hypertension in patients with pulmonary disease: COPD asthma. Chest. 2003;123:222–43. - PubMed

