The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals
- PMID: 18342009
- PMCID: PMC3606373
- DOI: 10.1016/j.immuni.2008.02.002
The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals
Abstract
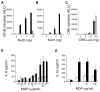
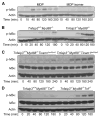
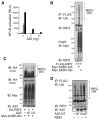
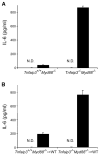
Muramyl dipeptide (MDP), a product of bacterial cell-wall peptidoglycan, activates innate immune cells by stimulating nucleotide-binding oligomerization domain containing 2 (NOD2) -dependent activation of the transcription factor NFkappaB and transcription of proinflammatory genes. A20 is a ubiquitin-modifying enzyme that restricts tumor necrosis factor (TNF) receptor and Toll-like receptor (TLR) -induced signals. We now show that MDP induces ubiquitylation of receptor- interacting protein 2 (RIP2) in primary macrophages. A20-deficient cells exhibit dramatically amplified responses to MDP, including increased RIP2 ubiquitylation, prolonged NFkappaB signaling, and increased production of proinflammatory cytokines. In addition, in vivo responses to MDP are exaggerated in A20-deficient mice and in chimeric mice bearing A20-deficient hematopoietic cells. These exaggerated responses occur independently of the TLR adaptors MyD88 and TRIF as well as TNF signals. These findings indicate that A20 directly restricts NOD2 induced signals in vitro and in vivo, and provide new insights into how these signals are physiologically restricted.
Figures


References
-
- Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

