Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability
- PMID: 18342310
- PMCID: PMC2805131
- DOI: 10.1016/j.expneurol.2008.01.016
Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability
Abstract
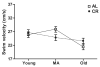
Caloric restriction (CR) is a daily reduction of total caloric intake without a decrease in micronutrients or disproportionate reduction of any one dietary component. CR can increase lifespan reliably in a wide range of species and appears to counteract some aspects of the aging process throughout the body. The effects on the brain are less clear, but moderate CR seems to attenuate age-related cognitive decline. Thus, we determined the effects of age and CR on key synaptic proteins in the CA3 region of the hippocampus and whether these changes were correlated with differences in behavior on a hippocampal-dependent learning and memory task. We observed an overall, age-related decline in the NR1, N2A and N2B subunits of the N-methyl-d-aspartate (NMDA)-type and the GluR1 and GluR2 subunits of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA)-type ionotropic glutamate receptors. Interestingly, we found that CR initially lowers the glutamate receptor subunit levels as compared to young AL animals, and then stabilizes the levels across lifespan. Synaptophysin, a presynaptic vesicle protein, showed a similar pattern. We also found that both CR and ad libitum (AL) fed animals exhibited age-related cognitive decline on the Morris water maze task. However, AL animals declined between young and middle age, and between middle age and old, whereas CR rats only declined between young and middle age. Thus, the decrease in key synaptic proteins in CA3 and cognitive decline occurring across lifespan are stabilized by CR. This age-related decrease and CR-induced stabilization are likely to affect CA3 synaptic plasticity and, as a result, hippocampal function.
Figures




References
-
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp Neurol. 2001;432:230–243. - PubMed
-
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 1995. pp. 443–495.
-
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. - PubMed
-
- Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, Ingram DK, Jucker M. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol. Aging. 1998;19:599–606. - PubMed
-
- Clark AS, Magnusson KR, Cotman CW. In vitro autoradiography of hippocampal excitatory amino acid binding in aged Fischer 344 rats: relationship to performance on the Morris water maze. Behav. Neurosci. 1992;106:324–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

