The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein
- PMID: 18342597
- PMCID: PMC2474773
- DOI: 10.1016/j.chom.2008.03.001
The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein
Abstract
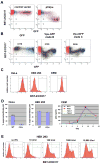
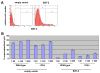
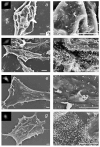
The HIV-1 accessory protein Vpu counteracts a host factor that restricts virion release from infected cells. Here we show that the interferon-induced cellular protein BST-2/HM1.24/CD317 is such a factor. BST-2 is downregulated from the cell surface by Vpu, and BST-2 is specifically expressed in cells that support the vpu phenotype. Exogenous expression of BST-2 inhibits HIV-1 virion release, while suppression of BST-2 relieves the requirement for Vpu. Downregulation of BST-2 requires both the transmembrane/ion channel domain and conserved serines in the cytoplasmic domain of Vpu. Endogenous BST-2 colocalizes with the HIV-1 structural protein Gag in endosomes and at the plasma membrane, suggesting that BST-2 traps virions within and on infected cells. The unusual structure of BST-2, which includes a transmembrane domain and a lumenal GPI anchor, may allow it to retain nascent enveloped virions on cellular membranes, providing a mechanism of viral restriction counteracted by a specific viral accessory protein.
Figures




References
-
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

