In-line system containing porous polymer monoliths for protein digestion with immobilized pepsin, peptide preconcentration and nano-liquid chromatography separation coupled to electrospray ionization mass spectroscopy
- PMID: 18342870
- PMCID: PMC2435401
- DOI: 10.1016/j.chroma.2008.02.075
In-line system containing porous polymer monoliths for protein digestion with immobilized pepsin, peptide preconcentration and nano-liquid chromatography separation coupled to electrospray ionization mass spectroscopy
Abstract
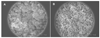
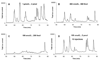
The use of two different monoliths located in capillaries for on-line protein digestion, preconcentration of peptides and their separation has been demonstrated. The first monolith was used as support for covalent immobilization of pepsin. This monolith with well-defined porous properties was prepared by in situ copolymerization of 2-vinyl-4,4-dimethylazlactone and ethylene dimethacrylate. The second, poly(lauryl methacrylate-co-ethylene dimethacrylate) monolith with a different porous structure served for the preconcentration of peptides from the digest and their separation in reversed-phase liquid chromatography mode. The top of the separation capillary was used as a preconcentrator, thus enabling the digestion of very dilute solutions of proteins in the bioreactor and increasing the sensitivity of the mass spectrometric detection of the peptides using a time-of-flight mass spectrometer with electrospray ionization. Myoglobin, albumin, and hemoglobin were digested to demonstrate feasibility of the concept of using the two monoliths in-line. Successive protein injections confirmed both the repeatability of the results and the ability to reuse the bioreactor for at least 20 digestions.
Figures

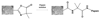




Similar articles
-
Dual-function microanalytical device by in situ photolithographic grafting of porous polymer monolith: integrating solid-phase extraction and enzymatic digestion for peptide mass mapping.Anal Chem. 2003 Oct 15;75(20):5328-35. doi: 10.1021/ac034108j. Anal Chem. 2003. PMID: 14710809
-
Integration of on-line protein digestion, peptide separation, and protein identification using pepsin-coated photopolymerized sol-gel columns and capillary electrophoresis/mass spectrometry.Anal Chem. 2004 Apr 1;76(7):1896-902. doi: 10.1021/ac035107u. Anal Chem. 2004. PMID: 15053649
-
Multidimensional system enabling deglycosylation of proteins using a capillary reactor with peptide-N-glycosidase F immobilized on a porous polymer monolith and hydrophilic interaction liquid chromatography-mass spectrometry of glycans.J Chromatogr A. 2009 Apr 10;1216(15):3252-9. doi: 10.1016/j.chroma.2009.02.036. Epub 2009 Feb 21. J Chromatogr A. 2009. PMID: 19268959
-
Immobilized trypsin systems coupled on-line to separation methods: recent developments and analytical applications.J Sep Sci. 2005 Jan;28(1):7-21. doi: 10.1002/jssc.200401941. J Sep Sci. 2005. PMID: 15688626 Review.
-
Porous polymer monoliths: an alternative to classical beads.Adv Biochem Eng Biotechnol. 2002;76:87-125. doi: 10.1007/3-540-45345-8_3. Adv Biochem Eng Biotechnol. 2002. PMID: 12126272 Review.
Cited by
-
Less common applications of monoliths: IV. Recent developments in immobilized enzyme reactors for proteomics and biotechnology.J Sep Sci. 2009 Mar;32(5-6):706-18. doi: 10.1002/jssc.200800641. J Sep Sci. 2009. PMID: 19194973 Free PMC article. Review.
-
Investigating Monoliths (Vinyl Azlactone-co-Ethylene Dimethacrylate) as a Support for Enzymes and Drugs, for Proteomics and Drug-Target Studies.Front Chem. 2019 Dec 3;7:835. doi: 10.3389/fchem.2019.00835. eCollection 2019. Front Chem. 2019. PMID: 31850321 Free PMC article.
-
Immobilized pepsin microreactor for rapid peptide mapping with nanoelectrospray ionization mass spectrometry.J Am Soc Mass Spectrom. 2015 Jan;26(1):194-7. doi: 10.1007/s13361-014-1015-8. Epub 2014 Nov 6. J Am Soc Mass Spectrom. 2015. PMID: 25374334
-
Functionalization of fibers using azlactone-containing polymers: layer-by-layer fabrication of reactive thin films on the surfaces of hair and cellulose-based materials.ACS Appl Mater Interfaces. 2010 May;2(5):1421-9. doi: 10.1021/am1000882. ACS Appl Mater Interfaces. 2010. PMID: 20402471 Free PMC article.
-
Immunoaffinity capillary electrophoresis as a powerful strategy for the quantification of low-abundance biomarkers, drugs, and metabolites in biological matrices.Electrophoresis. 2008 Aug;29(16):3259-78. doi: 10.1002/elps.200800058. Electrophoresis. 2008. PMID: 18646282 Free PMC article. Review.
References
-
- Girelli AM, Mattei E. J. Chromatogr. B. 2005;819:3. - PubMed
-
- Kallenberg AI, van Rantwijk F, Sheldon RA. Adv. Synth. Catal. 2005;347:905.
-
- Massolini G, Calleri E. J. Sep. Sci. 2005;28:7. - PubMed
-
- Giordano RC, Ribeiro MPA, Giordano RLC. Biotechnol. Adv. 2006;24:27. - PubMed
-
- Miyazaki M, Maeda H. Trends Biotechnol. 2006;24:463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

