Role of endocytosis and cathepsin-mediated activation in Nipah virus entry
- PMID: 18342904
- PMCID: PMC7103400
- DOI: 10.1016/j.virol.2008.02.019
Role of endocytosis and cathepsin-mediated activation in Nipah virus entry
Abstract
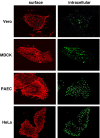
The recent discovery that the Nipah virus (NiV) fusion protein (F) is activated by endosomal cathepsin L raised the question if NiV utilize pH- and protease-dependent mechanisms of entry. We show here that the NiV receptor ephrin B2, virus-like particles and infectious NiV are internalized from the cell surface. However, endocytosis, acidic pH and cathepsin-mediated cleavage are not necessary for the initiation of infection of new host cells. Our data clearly demonstrate that proteolytic activation of the NiV F protein is required before incorporation into budding virions but not after virus entry.
Figures




Similar articles
-
Activation of the Nipah virus fusion protein in MDCK cells is mediated by cathepsin B within the endosome-recycling compartment.J Virol. 2012 Apr;86(7):3736-45. doi: 10.1128/JVI.06628-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278224 Free PMC article.
-
Ephrin-B2 expression critically influences Nipah virus infection independent of its cytoplasmic tail.Virol J. 2008 Dec 24;5:163. doi: 10.1186/1743-422X-5-163. Virol J. 2008. PMID: 19108727 Free PMC article.
-
Mutations in the G-H loop region of ephrin-B2 can enhance Nipah virus binding and infection.J Gen Virol. 2011 Sep;92(Pt 9):2142-2152. doi: 10.1099/vir.0.033787-0. Epub 2011 Jun 1. J Gen Virol. 2011. PMID: 21632558 Free PMC article.
-
Envelope-receptor interactions in Nipah virus pathobiology.Ann N Y Acad Sci. 2007 Apr;1102(1):51-65. doi: 10.1196/annals.1408.004. Ann N Y Acad Sci. 2007. PMID: 17470911 Free PMC article. Review.
-
Henipavirus membrane fusion and viral entry.Curr Top Microbiol Immunol. 2012;359:79-94. doi: 10.1007/82_2012_200. Curr Top Microbiol Immunol. 2012. PMID: 22427111 Review.
Cited by
-
Paramyxovirus Glycoproteins and the Membrane Fusion Process.Curr Clin Microbiol Rep. 2016 Sep;3(3):142-154. doi: 10.1007/s40588-016-0040-8. Epub 2016 Jul 5. Curr Clin Microbiol Rep. 2016. PMID: 28138419 Free PMC article.
-
Nipah virus fusion protein: Importance of the cytoplasmic tail for endosomal trafficking and bioactivity.Eur J Cell Biol. 2015 Jul-Sep;94(7-9):316-22. doi: 10.1016/j.ejcb.2015.05.005. Epub 2015 May 30. Eur J Cell Biol. 2015. PMID: 26059400 Free PMC article.
-
Characterization of the Bas-Congo virus glycoprotein and its function in pseudotyped viruses.J Virol. 2013 Sep;87(17):9558-68. doi: 10.1128/JVI.01183-13. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785218 Free PMC article.
-
Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein.PLoS Pathog. 2013;9(4):e1003309. doi: 10.1371/journal.ppat.1003309. Epub 2013 Apr 11. PLoS Pathog. 2013. PMID: 23593008 Free PMC article.
-
Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4904-12. doi: 10.1073/pnas.1302334110. Epub 2013 Nov 4. Proc Natl Acad Sci U S A. 2013. PMID: 24191014 Free PMC article.
References
-
- Adams R.H. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin. Cell Dev. Biol. 2002;13:55–60. - PubMed
-
- Cantin C., Holguera J., Ferreira L., Villar E., Munoz-Barroso I. Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J. Gen. Virol. 2007;88:559–569. - PubMed
-
- Chen C., Vincent O., Jin J., Weisz O.A., Montelaro R.C. Functions of early (AP-2) and late (AIP1/ALIX) endocytic proteins in equine infectious anemia virus budding. J. Biol. Chem. 2005;280:40474–40480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

