Role of endocytosis and cathepsin-mediated activation in Nipah virus entry
- PMID: 18342904
- PMCID: PMC7103400
- DOI: 10.1016/j.virol.2008.02.019
Role of endocytosis and cathepsin-mediated activation in Nipah virus entry
Abstract
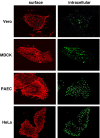
The recent discovery that the Nipah virus (NiV) fusion protein (F) is activated by endosomal cathepsin L raised the question if NiV utilize pH- and protease-dependent mechanisms of entry. We show here that the NiV receptor ephrin B2, virus-like particles and infectious NiV are internalized from the cell surface. However, endocytosis, acidic pH and cathepsin-mediated cleavage are not necessary for the initiation of infection of new host cells. Our data clearly demonstrate that proteolytic activation of the NiV F protein is required before incorporation into budding virions but not after virus entry.
Figures




References
-
- Adams R.H. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin. Cell Dev. Biol. 2002;13:55–60. - PubMed
-
- Cantin C., Holguera J., Ferreira L., Villar E., Munoz-Barroso I. Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J. Gen. Virol. 2007;88:559–569. - PubMed
-
- Chen C., Vincent O., Jin J., Weisz O.A., Montelaro R.C. Functions of early (AP-2) and late (AIP1/ALIX) endocytic proteins in equine infectious anemia virus budding. J. Biol. Chem. 2005;280:40474–40480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

