The micro-architecture of the cerebral cortex: functional neuroimaging models and metabolism
- PMID: 18343162
- PMCID: PMC4348032
- DOI: 10.1016/j.neuroimage.2007.12.051
The micro-architecture of the cerebral cortex: functional neuroimaging models and metabolism
Abstract
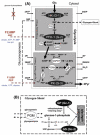
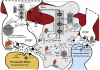
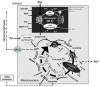
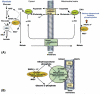
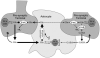
In order to interpret/integrate data obtained with different functional neuroimaging modalities (e.g. fMRI, EEG/MEG, PET/SPECT, fNIRS), forward-generative models of a diversity of brain mechanisms at the mesoscopic level are considered necessary. For the cerebral cortex, the brain structure with possibly the most relevance for functional neuroimaging, a variety of such biophysical models has been proposed over the last decade. The development of technological tools to investigate in vitro the physiological, anatomical and biochemical principles at the microscopic scale in comparative studies formed the basis for such theoretical progresses. However, with the most recent introduction of systems to record electrical (e.g. miniaturized probes chronically/acutely implantable in the brain), optical (e.g. two-photon laser scanning microscopy) and atomic nuclear spectral (e.g. nuclear magnetic resonance spectroscopy) signals using living laboratory animals, the field is receiving even greater attention. Major advances have been achieved by combining such sophisticated recording systems with new experimental strategies (e.g. transgenic/knock-out animals, high resolution stereotaxic manipulation systems for probe-guidance and cellular-scale chemical-delivery). Theoreticians may now be encouraged to re-consider previously formulated mesoscopic level models in order to incorporate important findings recently made at the microscopic scale. In this series of reviews, we summarize the background at the microscopic scale, which we suggest will constitute the foundations for upcoming representations at the mesoscopic level. In this first part, we focus our attention on the nerve ending particles in order to summarize basic principles and mechanisms underlying cellular metabolism in the cerebral cortex. It will be followed by two parts highlighting major features in its organization/working-principles to regulate both cerebral blood circulation and neuronal activity, respectively. Contemporary theoretical models for functional neuroimaging will be revised in the fourth part, with particular emphasis in their applications, advantages/limitations and future prospects.
Figures

Similar articles
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
-
Energetic basis of brain activity: implications for neuroimaging.Trends Neurosci. 2004 Aug;27(8):489-95. doi: 10.1016/j.tins.2004.06.005. Trends Neurosci. 2004. PMID: 15271497 Review.
-
In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine.J Nutr. 2001 Sep;131(9 Suppl):2498S-504S; discussion 2523S-4S. doi: 10.1093/jn/131.9.2498S. J Nutr. 2001. PMID: 11533301 Review.
-
In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics.Philos Trans R Soc Lond B Biol Sci. 1999 Jul 29;354(1387):1165-77. doi: 10.1098/rstb.1999.0472. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10466144 Free PMC article. Review.
-
Characterization of energy and neurotransmitter metabolism in cortical glutamatergic neurons derived from human induced pluripotent stem cells: A novel approach to study metabolism in human neurons.Neurochem Int. 2017 Jun;106:48-61. doi: 10.1016/j.neuint.2017.02.010. Epub 2017 Feb 24. Neurochem Int. 2017. PMID: 28237843
Cited by
-
Functional importance of the astrocytic glycogen-shunt and glycolysis for maintenance of an intact intra/extracellular glutamate gradient.Neurotox Res. 2010 Jul;18(1):94-9. doi: 10.1007/s12640-010-9171-5. Epub 2010 Mar 20. Neurotox Res. 2010. PMID: 20306167 Review.
-
Quantitative basis for neuroimaging of cortical laminae with calibrated functional MRI.Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):15115-20. doi: 10.1073/pnas.1307154110. Epub 2013 Aug 26. Proc Natl Acad Sci U S A. 2013. PMID: 23980158 Free PMC article.
-
Neuroenergetics at the brain-mind interface: a conceptual approach.Cogn Process. 2014 Aug;15(3):297-306. doi: 10.1007/s10339-014-0609-1. Epub 2014 Mar 17. Cogn Process. 2014. PMID: 24633631 Review.
-
Concepts of connectivity and human epileptic activity.Front Syst Neurosci. 2011 Mar 22;5:12. doi: 10.3389/fnsys.2011.00012. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21472027 Free PMC article.
-
The Brain Connectivity Workshops: moving the frontiers of computational systems neuroscience.Neuroimage. 2008 Aug 1;42(1):1-9. doi: 10.1016/j.neuroimage.2008.04.167. Epub 2008 Apr 20. Neuroimage. 2008. PMID: 18511300 Free PMC article.
References
-
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. - PubMed
-
- Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004;6:45–51. - PubMed
-
- Ames CNS energy metabolism as related to function. Brains Res. Rev. 2000;34:42–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

