Antiparasitic compounds that target DNA
- PMID: 18343228
- PMCID: PMC2515095
- DOI: 10.1016/j.biochi.2008.02.017
Antiparasitic compounds that target DNA
Abstract
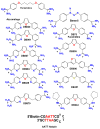
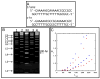
Designed, synthetic heterocyclic diamidines have excellent activity against eukaryotic parasites that cause diseases such as sleeping sickness and leishmania and adversely affect millions of people each year. The most active compounds bind specifically and strongly in the DNA minor groove at AT sequences. The compounds enter parasite cells rapidly and appear first in the kinetoplast that contains the mitochondrial DNA of the parasite. With time the compounds are also generally seen in the cell nucleus but are not significantly observed in the cytoplasm. The kinetoplast decays over time and disappears from the mitochondria of treated cells. At this point the compounds begin to be observed in other regions of the cell, such as the acidocalcisomes. The cells typically die in 24-48h after treatment. Active compounds appear to selectively target extended AT sequences and induce changes in kinetoplast DNA minicircles that cause a synergistic destruction of the catenated kinetoplast DNA network and cell death.
Figures







References
-
- Watkins BM. Drugs for the control of parasitic diseases: current status and development. Trends Parasitol. 2003;19:477–478. - PubMed
-
- World Health Organization (WHO) Research Results: Nos. 1, 6. and 7; African trypanosomiasis; Fact Sheet Number 259. World Health Organization Publications; Geneva: 2001.
-
- Vincendeau P, Bouteille B. Immunology and immunopathology of African trypanosomiasis. An Acad Bras Cienc. 2006;78:645–665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

