Acute and chronic heroin dependence in mice: contribution of opioid and excitatory amino acid receptors
- PMID: 18343363
- PMCID: PMC3627364
- DOI: 10.1016/j.ejphar.2008.02.035
Acute and chronic heroin dependence in mice: contribution of opioid and excitatory amino acid receptors
Abstract
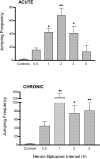
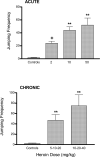
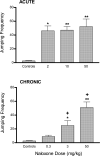
Opioid and excitatory amino acid receptors contribute to morphine dependence, but there are no studies of their role in heroin dependence. Thus, mice injected with acute or chronic heroin doses in the present study were pretreated with one of the following selective antagonists: 7-benzylidenenaltrexone (BNTX), naltriben (NTB), nor-binaltorphimine (nor-BNI; delta1, delta2, and kappa opioid receptors, respectively), MK-801, or LY293558 (NMDA and AMPA excitatory amino acid receptors, respectively). Naloxone-precipitated withdrawal jumping frequency, shown here to be a reliable index of heroin dependence magnitude, was reduced by BNTX or NTB in mice injected with both acute and chronic heroin doses. In contrast, nor-BNI did not alter jumping frequencies in mice injected with an acute heroin dose but significantly increased them in mice receiving chronic heroin injections. Continuous MK-801 or LY293558 infusion, but not injection, reduced jumping frequencies during withdrawal from acute heroin treatment. Their delivery by injection was nonetheless effective against chronic heroin dependence, suggesting mechanisms not simply attributable to NMDA or AMPA blockade. These data indicate that whereas delta1, delta2, NMDA, and AMPA receptors enable acute and chronic heroin dependence, kappa receptor activity limits the dependence liability of chronic heroin. With the exception of delta1 receptors, the apparent role of these receptors to heroin dependence is consistent with their contribution to morphine dependence, indicating that there is substantial physiological commonality underlying dependence to both heroin and morphine. The ability of kappa receptor blockade to differentially alter acute and chronic dependence supports previous assertions from studies with other opioids that acute and chronic opioid dependence are, at least in part, mechanistically distinct. Elucidating the substrates contributing to heroin dependence, and identifying their similarities and differences with those of other opioids such as morphine, may yield effective treatment strategies to the problem of heroin dependency.
Figures
Similar articles
-
Naloxone rapidly evokes endogenous kappa opioid receptor-mediated hyperalgesia in naïve mice pretreated briefly with GM1 ganglioside or in chronic morphine-dependent mice.Brain Res. 2007 Sep 5;1167:31-41. doi: 10.1016/j.brainres.2007.06.058. Epub 2007 Jul 14. Brain Res. 2007. PMID: 17692296
-
Involvement of delta 1 and delta 2 opioid receptor subtypes in the development of physical dependence on morphine in mice.Pharmacol Biochem Behav. 1997 May-Jun;57(1-2):293-9. doi: 10.1016/s0091-3057(96)00319-x. Pharmacol Biochem Behav. 1997. PMID: 9164585
-
The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence.Brain Res. 1997 Dec 5;778(1):120-6. doi: 10.1016/s0006-8993(97)00985-2. Brain Res. 1997. PMID: 9462883
-
The role of functional postsynaptic NMDA receptors in the central nucleus of the amygdala in opioid dependence.Vitam Horm. 2010;82:145-66. doi: 10.1016/S0083-6729(10)82008-4. Vitam Horm. 2010. PMID: 20472137 Free PMC article. Review.
-
It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system.Front Pharmacol. 2014 May 27;5:116. doi: 10.3389/fphar.2014.00116. eCollection 2014. Front Pharmacol. 2014. PMID: 24904419 Free PMC article. Review.
Cited by
-
In vivo characterization of the opioid antagonist nalmefene in mice.Life Sci. 2010 Apr 10;86(15-16):624-30. doi: 10.1016/j.lfs.2010.02.013. Epub 2010 Feb 14. Life Sci. 2010. PMID: 20159022 Free PMC article.
-
Heroin Addiction Induces Axonal Transport Dysfunction in the Brain Detected by In Vivo MRI.Neurotox Res. 2022 Aug;40(4):1070-1085. doi: 10.1007/s12640-022-00533-3. Epub 2022 Jun 27. Neurotox Res. 2022. PMID: 35759084
-
All Hands on Deck: We Need Multiple Approaches To Uncover the Neuroscience behind the Opioid Overdose Crisis.ACS Chem Neurosci. 2023 Jun 7;14(11):1921-1929. doi: 10.1021/acschemneuro.2c00818. Epub 2023 May 9. ACS Chem Neurosci. 2023. PMID: 37159430 Free PMC article. Review.
-
A survey of acute and chronic heroin dependence in ten inbred mouse strains: evidence of genetic correlation with morphine dependence.Pharmacol Biochem Behav. 2008 Sep;90(3):447-52. doi: 10.1016/j.pbb.2008.03.030. Epub 2008 Apr 4. Pharmacol Biochem Behav. 2008. PMID: 18472145 Free PMC article.
-
Ultrastructural relationship between the AMPA-GluR2 receptor subunit and the mu-opioid receptor in the mouse central nucleus of the amygdala.Exp Neurol. 2011 Jan;227(1):149-58. doi: 10.1016/j.expneurol.2010.10.010. Epub 2010 Oct 21. Exp Neurol. 2011. PMID: 20970421 Free PMC article.
References
-
- Beart PM, Lodge D. Chronic administration of MK-801 and the NMDA receptor: further evidence for reduced sensitivity of the primary acceptor site from studies with the cortical wedge preparation. J. Pharm. Pharmacol. 1990;42:354–355. - PubMed
-
- Bettler B, Mulle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Breivik H. Opioids in cancer and chronic non-cancer pain therapy - indications and controversies. Acta Anesthes. Scandinavia. 2001;45:1059–1066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

