Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein
- PMID: 18343814
- PMCID: PMC2423264
- DOI: 10.1074/jbc.M800747200
Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein
Abstract
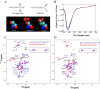
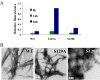
alpha-Synuclein (alpha-syn) phosphorylation at serine 129 is characteristic of Parkinson disease (PD) and related alpha-synulceinopathies. However, whether phosphorylation promotes or inhibits alpha-syn aggregation and neurotoxicity in vivo remains unknown. This understanding is critical for elucidating the role of alpha-syn in the pathogenesis of PD and for development of therapeutic strategies for PD. To better understand the structural and molecular consequences of Ser-129 phosphorylation, we compared the biochemical, structural, and membrane binding properties of wild type alpha-syn to those of the phosphorylation mimics (S129E, S129D) as well as of in vitro phosphorylated alpha-syn using a battery of biophysical techniques. Our results demonstrate that phosphorylation at Ser-129 increases the conformational flexibility of alpha-syn and inhibits its fibrillogenesis in vitro but does not perturb its membrane-bound conformation. In addition, we show that the phosphorylation mimics (S129E/D) do not reproduce the effect of phosphorylation on the structural and aggregation properties of alpha-syn in vitro. Our findings have significant implications for current strategies to elucidate the role of phosphorylation in modulating protein structure and function in health and disease and provide novel insight into the underlying mechanisms that govern alpha-syn aggregation and toxicity in PD and related alpha-synulceinopathies.
Figures





References
-
- Trojanowski, J. Q., and Lee, V. M. (2003) Ann. N. Y. Acad. Sci. 991107 -110 - PubMed
-
- Abeliovich, A., Schmitz, Y., Farinas, I., Choi-Lundberg, D., Ho, W. H., Castillo, P. E., Shinsky, N., Verdugo, J. M., Armanini, M., Ryan, A., Hynes, M., Phillips, H., Sulzer, D., and Rosenthal, A. (2000) Neuron 25239 -252 - PubMed
-
- Cooper, A. A., Gitler, A. D., Cashikar, A., Haynes, C. M., Hill, K. J., Bhullar, B., Liu, K., Xu, K., Strathearn, K. E., Liu, F., Cao, S., Caldwell, K. A., Caldwell, G. A., Marsischky, G., Kolodner, R. D., Labaer, J., Rochet, J. C., Bonini, N. M., and Lindquist, S. (2006) Science 313324 -328 - PMC - PubMed
-
- Kahle, P. J., Neumann, M., Ozmen, L., and Haass, C. (2000) Ann. N. Y. Acad. Sci. 920 33-41 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

