Glial cell line-derived neurotrophic factor reverses ethanol-mediated increases in tyrosine hydroxylase immunoreactivity via altering the activity of heat shock protein 90
- PMID: 18343820
- PMCID: PMC2442340
- DOI: 10.1074/jbc.M706216200
Glial cell line-derived neurotrophic factor reverses ethanol-mediated increases in tyrosine hydroxylase immunoreactivity via altering the activity of heat shock protein 90
Abstract
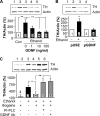
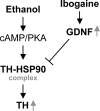
We previously found that glial cell line-derived neurotrophic factor (GDNF) in the midbrain ventral tegmental area (VTA) negatively regulates alcohol drinking (He, D. Y., McGough, N. N., Ravindranathan, A., Jeanblanc, J., Logrip, M. L., Phamluong, K., Janak, P. H., and Ron, D. (2005) J. Neurosci. 25, 619-628). Several studies suggest a role for GDNF in the regulation of tyrosine hydroxylase (TH) levels in the midbrain (Georgievska, B., Kirik, D., and Bjorklund, A. (2004) J. Neurosci. 24, 6437-6445). Up-regulation of TH levels has been reported as a hallmark of biochemical adaptations to in vivo chronic exposure to drugs of abuse, including ethanol (Ortiz, J., Fitzgerald, L. W., Charlton, M., Lane, S., Trevisan, L., Guitart, X., Shoemaker, W., Duman, R. S., and Nestler, E. J. (1995) Synapse 21, 289-298). We hypothesized that GDNF plays an important role in regulating prolonged ethanol-mediated increases in TH protein levels. Using the SH-SY5Y dopaminergic-like cell line, we found that the increase in TH levels in the presence of ethanol required the activation of the cAMP/PKA pathway and was reversed by GDNF. Ethanol treatment did not alter the mRNA level or protein translation of TH, but enhanced the stability of the protein that was decreased by GDNF. Interestingly, we observed that ethanol treatment resulted in an increase in TH association with the chaperone heat shock protein (HSP90) that was mediated by the cAMP/PKA pathway and inhibited by GDNF. Taken together, these data suggest that prolonged ethanol exposure leads to increased association of TH and HSP90 via the cAMP/PKA pathway, resulting in the stabilization and subsequent accumulation of TH. GDNF reverses this ethanol-mediated adaptation by inhibiting the interaction of TH with HSP90.
Figures
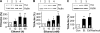





Similar articles
-
Glial cell line-derived neurotrophic factor up-regulates the expression of tyrosine hydroxylase gene in human neuroblastoma cell lines.J Neurochem. 2002 Aug;82(4):801-8. doi: 10.1046/j.1471-4159.2002.00993.x. J Neurochem. 2002. PMID: 12358785
-
An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway.J Neurosci. 2006 Mar 22;26(12):3335-44. doi: 10.1523/JNEUROSCI.5010-05.2006. J Neurosci. 2006. PMID: 16554484 Free PMC article.
-
Signaling of glial cell line-derived neurotrophic factor and its receptor GFRα1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease.J Neuropathol Exp Neurol. 2011 Sep;70(9):736-47. doi: 10.1097/NEN.0b013e31822830e5. J Neuropathol Exp Neurol. 2011. PMID: 21865882
-
GDNF and alcohol use disorder.Addict Biol. 2019 May;24(3):335-343. doi: 10.1111/adb.12628. Epub 2018 May 4. Addict Biol. 2019. PMID: 29726054 Free PMC article. Review.
-
GDNF and addiction.Rev Neurosci. 2005;16(4):277-85. doi: 10.1515/revneuro.2005.16.4.277. Rev Neurosci. 2005. PMID: 16519005 Review.
Cited by
-
Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF expression and ethanol self-administration.Addict Biol. 2010 Oct;15(4):424-33. doi: 10.1111/j.1369-1600.2010.00251.x. Addict Biol. 2010. PMID: 21040239 Free PMC article.
-
cAMP-mediated stimulation of tyrosine hydroxylase mRNA translation is mediated by polypyrimidine-rich sequences within its 3'-untranslated region and poly(C)-binding protein 2.Mol Pharmacol. 2009 Oct;76(4):872-83. doi: 10.1124/mol.109.057596. Epub 2009 Jul 20. Mol Pharmacol. 2009. PMID: 19620256 Free PMC article.
-
Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors.J Neurosci. 2010 May 5;30(18):6236-46. doi: 10.1523/JNEUROSCI.0567-10.2010. J Neurosci. 2010. PMID: 20445049 Free PMC article.
-
GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence.Alcohol Clin Exp Res. 2009 Jun;33(6):1012-24. doi: 10.1111/j.1530-0277.2009.00922.x. Epub 2009 Mar 19. Alcohol Clin Exp Res. 2009. PMID: 19302086 Free PMC article.
-
Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor.Alcohol. 2009 Feb;43(1):35-43. doi: 10.1016/j.alcohol.2008.12.001. Alcohol. 2009. PMID: 19185208 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

