MADS-box transcription factor mig1 is required for infectious growth in Magnaporthe grisea
- PMID: 18344407
- PMCID: PMC2394974
- DOI: 10.1128/EC.00009-08
MADS-box transcription factor mig1 is required for infectious growth in Magnaporthe grisea
Abstract
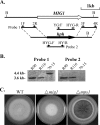
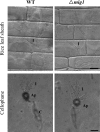
Magnaporthe grisea is a model fungus for studying fungus-plant interactions. Two mitogen-activated protein (MAP) kinase genes, PMK1 and MPS1, have been implicated in regulating plant infection processes in M. grisea. However, transcription factors activated by these MAP kinases are not well studied. In this study we functionally characterized the MIG1 gene that encodes a MADS-box transcription factor homologous to Saccharomyces cerevisiae Rlm1. In yeast two-hybrid assays, MIG1 interacts with MPS1, suggesting that MIG1 may function downstream from the MPS1 pathway. The mig1 deletion mutant had a normal growth rate and formed melanized appressoria, but it was nonpathogenic and failed to infect rice leaves through wounds. Appressoria formed by the mig1 mutant developed penetration pegs and primary infectious hyphae, but further differentiation of the secondary infectious hyphae inside live plant cells was blocked. However, the mig1 mutant formed infectious hypha-like structures in heat-killed plant cells or cellophane membranes. In transformants expressing the MIG1-GFP fusion, green fluorescent protein (GFP) signals were not detectable in vegetative hyphae and conidiophores. Mig1-GFP was localized to nuclei in conidia, appressoria, and infectious hyphae. Deletion of the MADS box had no effect on the expression and localization of the MIG1-GFP fusion but eliminated its ability to complement the mig1 mutant. These results suggest that MIG1 may be required for overcoming plant defense responses and the differentiation of secondary infectious hyphae in live plant cells. The MADS-box domain is essential for the function of MIG1 but dispensable for its nuclear localization, which may be associated with the activation of MIG1 by MPS1 during conidiation and plant infection.
Figures





References
-
- Bourett, T. M., and R. J. Howard. 1990. In vitro development of penetration structures in the rice blast fungus Magnaporthe grisea. Can. J. Bot. 68329-342.
-
- Catlett, N. L., B. Lee, O. C. Yoder, and B. G. Turgeon. 2003. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl. 509-11.
-
- Chao, C. C. T., and A. H. Ellingboe. 1991. Selection for mating competence in Magnaporthe grisea pathogenic to rice. Can. J. Bot. 692130-2134.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

