Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2
- PMID: 18344974
- PMCID: PMC2288760
- DOI: 10.1038/embor.2008.28
Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2
Abstract
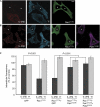
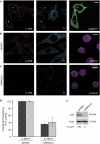
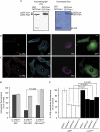
There are several endocytic pathways, which are either dependent on or independent of clathrin. This study focuses on a poorly characterized mechanism-clathrin- and caveolae-independent endocytosis-used by the interleukin-2 receptor beta (IL-2R beta). We address the question of its regulation in comparison with the clathrin-dependent pathway. First, we show that Ras-related C3 botulinum toxin substrate 1 (Rac1) is specifically required for IL-2R beta entry, and we identify p21-activated kinases (Paks) as downstream targets. By RNA interference, we show that Pak1 and Pak2 are both necessary for IL-2R beta uptake, in contrast to the clathrin-dependent route. We observe that cortactin, a partner of actin and dynamin-two essential endocytic factors-is required for IL-2R beta uptake. Furthermore, we find that cortactin acts downstream from Paks, suggesting control of its function by these kinases. Thus, we describe a cascade composed of Rac1, Paks and cortactin specifically regulating IL-2R beta internalization. This study indicates Paks as the first specific regulators of the clathrin-independent endocytosis pathway.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Burridge K, Doughman R (2006) Front and back by Rho and Rac. Nat Cell Biol 8: 781–782 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

