Identification of oxadiazoles as new drug leads for the control of schistosomiasis
- PMID: 18345010
- PMCID: PMC2700043
- DOI: 10.1038/nm1737
Identification of oxadiazoles as new drug leads for the control of schistosomiasis
Abstract
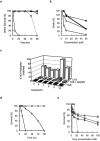
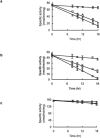
Treatment for schistosomiasis, which is responsible for more than 280,000 deaths annually, depends almost exclusively on praziquantel. Millions of people are treated annually with praziquantel, and drug-resistant parasites thus are likely to evolve. Phosphinic amides and oxadiazole 2-oxides, identified from a quantitative high-throughput screen, were shown to inhibit a parasite enzyme, thioredoxin glutathione reductase (TGR), with activities in the low micromolar to low nanomolar range. Incubation of parasites with these compounds led to rapid inhibition of TGR activity and parasite death. The activity of the oxadiazole 2-oxides was associated with a donation of nitric oxide. Treatment of schistosome-infected mice with 4-phenyl-1,2,5-oxadiazole-3-carbonitrile-2-oxide led to marked reductions in worm burdens from treatments against multiple parasite stages and egg-associated pathologies. The compound was active against the three major schistosome species infecting humans. These protective effects exceed benchmark activity criteria set by the World Health Organization for lead compound development for schistosomiasis.
Figures


Comment in
-
New drugs for an ancient parasite.Nat Med. 2008 Apr;14(4):365-7. doi: 10.1038/nm0408-365. Nat Med. 2008. PMID: 18391931 No abstract available.
References
-
- Hotez PJ, et al. Control of neglected tropical diseases. New Eng. J. Med. 2007;357:1018–1027. - PubMed
-
- van der Werf MJ, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. - PubMed
-
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. - PubMed
-
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6:411–425. - PubMed
-
- Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Curr. Opin. Infect. Dis. 2006;19:577–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

