Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16
- PMID: 18345032
- PMCID: PMC7100519
- DOI: 10.1038/onc.2008.53
Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16
Abstract
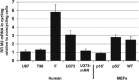
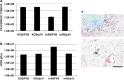
Oncolytic herpes simplex viruses (HSVs), in clinical trials for the treatment of malignant gliomas, are assumed to be selective for tumor cells because their replication is strongly attenuated in quiescent cells, but not in cycling cells. Oncolytic selectivity is thought to occur because mutations in viral ICP6 (encoding a viral ribonucleotide reductase function) and/or gamma34.5 function are respectively complemented by mammalian ribonucleotide reductase and GADD34, whose genes are expressed in cycling cells. However, it is estimated that only 5-15% of malignant glioma cells are in mitosis at any one time. Therefore, effective replication of HSV oncolytic viruses might be limited to a subpopulation of tumor cells, since at any one time the majority of tumor cells would not be cycling. However, we report that an HSV with defective ICP6 function replicates in quiescent cultured murine embryonic fibroblasts obtained from mice with homozygous p16 deletions. Furthermore, intracranial inoculation of this virus into the brains of p16-/- mice provides evidence of viral replication that does not occur when the virus is injected into the brains of wild-type mice. These approaches provide in vitro and in vivo evidence that ICP6-negative HSVs are 'molecularly targeted,' because they replicate in quiescent tumor cells carrying specific oncogene deletions, independent of cell cycle status.
Figures

References
-
- Costello JF, Berger MS, Huang HS, Cavenee WK. Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res. 1996;56:2405–2410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

