Added astroglia promote greater synapse density and higher activity in neuronal networks
- PMID: 18345351
- PMCID: PMC2267743
- DOI: 10.1017/S1740925X07000440
Added astroglia promote greater synapse density and higher activity in neuronal networks
Abstract
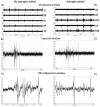
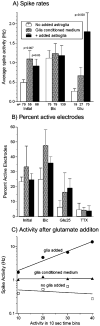
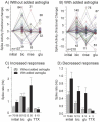
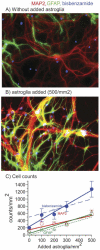
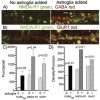
Astroglia are known to potentiate individual synapses, but their contribution to networks is unclear. Here we examined the effect of adding either astroglia or media conditioned by astroglia on entire networks of rat hippocampal neurons cultured on microelectrode arrays. Added astroglia increased spontaneous spike rates nearly two-fold and glutamate-stimulated spiking by six-fold, with desensitization eliminated for bath addition of 25 microM glutamate. Astrocyte-conditioned medium partly mimicked the effects of added astroglia. Bursting behavior was largely unaffected by added astroglia except with added glutamate. Addition of the GABA(A) receptor antagonist bicuculline also increased spike rates but with more subtle differences between networks without or with added astroglia. This indicates that networks without added astroglia were inhibited greatly. In all conditions, the log-log distribution of spike rates fit well to linear distributions over three orders of magnitude. Networks with added astroglia shifted consistently toward higher spike rates. Immunostaining for GFAP revealed a linear increase with added astroglia, which also increased neuronal survival. The increased spike rates with added astroglia correlated with a 1.7-fold increase in immunoreactive synaptophysin puncta, and increases of six-fold for GABA(Abeta), two-fold for NMDA-R1 and two-fold for Glu-R1 puncta, with receptor clustering that indicated synaptic scaling. Together, these results indicate that added astroglia increase the density of synapses and receptors, and facilitate higher spike rates for many elements in the network. These effects are reproduced by glia-conditioned media, with the exception of glutamate-mediated transmission.
Keywords: multielectrode array; neuron–glia cell culture; serum-free culture; synaptic scaling; synaptogenesis.
Figures


References
-
- Abney ER, Bartlett PP, Raff MC. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Developmental Biology. 1981;83:301–310. - PubMed
-
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. - PubMed
-
- Barbin G, Pollard H, Gaiarsa JL, Ben Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neuroscience Letters. 1993;152:150–154. - PubMed
-
- Barker JL, Bahar TN, Ma W, Maric D, Maric I. GABA emerges as a developmental signal during neurogenesis of the rat central nervous system. In: Martin DL, Olsen RW, editors. GABA in the Nervous System. Lippincott Williams and Wilkens; 2000. pp. 245–263.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
