Ca2+ -dependent regulation of phototransduction
- PMID: 18346093
- PMCID: PMC4118144
- DOI: 10.1111/j.1751-1097.2008.00323.x
Ca2+ -dependent regulation of phototransduction
Abstract
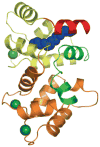
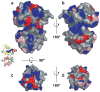
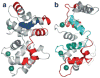
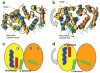
Photon absorption by rhodopsin triggers the phototransduction signaling pathway that culminates in degradation of cGMP, closure of cGMP-gated ion channels and hyperpolarization of the photoreceptor membrane. This process is accompanied by a decrease in free Ca(2+) concentration in the photoreceptor cytosol sensed by Ca(2+)-binding proteins that modulate phototransduction and activate the recovery phase to reestablish the photoreceptor dark potential. Guanylate cyclase-activating proteins (GCAPs) belong to the neuronal calcium sensor (NCS) family and are responsible for activating retinal guanylate cyclases (retGCs) at low Ca(2+) concentrations triggering synthesis of cGMP and recovery of the dark potential. Here we review recent structural insight into the role of the N-terminal myristoylation in GCAPs and compare it to other NCS family members. We discuss previous studies identifying regions of GCAPs important for retGC1 regulation in the context of the new structural data available for myristoylated GCAP1. In addition, we present a hypothetical model for the Ca(2+)-triggered conformational change in GCAPs and retGC1 regulation. Finally, we briefly discuss the involvement of mutant GCAP1 proteins in the etiology of retinal degeneration as well as the importance of other Ca(2+) sensors in the modulation of phototransduction.
Figures

References
-
- Palczewski K, Polans AS, Baehr W, Ames JB. Ca2+-binding proteins in the retina: Structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–350. - PubMed
-
- Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends Neurosci. 1996;19:547–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

