A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and beta-cell glucose sensing is associated with type 2 diabetes in adults
- PMID: 18346985
- PMCID: PMC6101196
- DOI: 10.2337/db07-1547
A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and beta-cell glucose sensing is associated with type 2 diabetes in adults
Abstract
Objective: ATP-sensitive K(+) channels (K(ATP) channels) link glucose metabolism to the electrical activity of the pancreatic beta-cell to regulate insulin secretion. Mutations in either the Kir6.2 or sulfonylurea receptor (SUR) 1 subunit of the channel have previously been shown to cause neonatal diabetes. We describe here an activating mutation in the ABCC8 gene, encoding SUR1, that is associated with the development of type 2 diabetes only in adults.
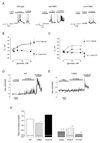
Research design and methods: Recombinant K(ATP) channel subunits were expressed using pIRES2-based vectors in human embryonic kidney (HEK) 293 or INS1(832/13) cells and the subcellular distribution of c-myc-tagged SUR1 channels analyzed by confocal microscopy. K(ATP) channel activity was measured in inside-out patches and plasma membrane potential in perforated whole-cell patches. Cytoplasmic [Ca(2+)] was imaged using Fura-Red.
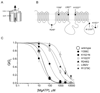
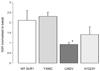
Results: A mutation in ABCC8/SUR1, leading to a Y356C substitution in the seventh membrane-spanning alpha-helix, was observed in a patient diagnosed with hyperglycemia at age 39 years and in two adult offspring with impaired insulin secretion. Single K(ATP) channels incorporating SUR1-Y356C displayed lower sensitivity to MgATP (IC(50) = 24 and 95 micromol/l for wild-type and mutant channels, respectively). Similar effects were observed in the absence of Mg(2+), suggesting an allosteric effect via associated Kir6.2 subunits. Overexpression of SUR1-Y356C in INS1(832/13) cells impaired glucose-induced cell depolarization and increased in intracellular free Ca(2+) concentration, albeit more weakly than neonatal diabetes-associated SUR1 mutants.
Conclusions: An ABCC8/SUR1 mutation with relatively minor effects on K(ATP) channel activity and beta-cell glucose sensing causes diabetes in adulthood. These data suggest a close correlation between altered SUR1 properties and clinical phenotype.
Figures


References
-
- Ashcroft FM. K(ATP) channels and insulin secretion: a key role in health and disease. Biochem Soc Trans. 2006;34:243–246. - PubMed
-
- Rutter GA. Visualising insulin secretion. The Minkowski Lecture 2004. Diabetologia. 2004;47:1861–1872. - PubMed
-
- Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. - PubMed
-
- Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81:375–382. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

