Identification of TbpA residues required for transferrin-iron utilization by Neisseria gonorrhoeae
- PMID: 18347046
- PMCID: PMC2346694
- DOI: 10.1128/IAI.00020-08
Identification of TbpA residues required for transferrin-iron utilization by Neisseria gonorrhoeae
Abstract
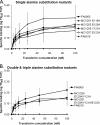
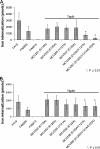
Neisseria gonorrhoeae requires iron for survival in the human host and therefore expresses high-affinity receptors for iron acquisition from host iron-binding proteins. The gonococcal transferrin-iron uptake system is composed of two transferrin binding proteins, TbpA and TbpB. TbpA is a TonB-dependent, outer membrane transporter critical for iron acquisition, while TbpB is a surface-exposed lipoprotein that increases the efficiency of iron uptake. The precise mechanism by which TbpA mediates iron acquisition has not been elucidated; however, the process is distinct from those of characterized siderophore transporters. Similar to these TonB-dependent transporters, TbpA is proposed to have two distinct domains, a beta-barrel and a plug domain. We hypothesize that the TbpA plug coordinates iron and therefore potentially functions in multiple steps of transferrin-mediated iron acquisition. To test this hypothesis, we targeted a conserved motif within the TbpA plug domain and generated single, double, and triple alanine substitution mutants. Mutagenized TbpAs were expressed on the gonococcal cell surface and maintained wild-type transferrin binding affinity. Single alanine substitution mutants internalized iron at wild-type levels, while the double and triple mutants showed a significant decrease in iron uptake. Moreover, the triple alanine substitution mutant was unable to grow on transferrin as a sole iron source; however, expression of TbpB compensated for this defect. These data indicate that the conserved motif between residues 120 and 122 of the TbpA plug domain is critical for transferrin-iron utilization, suggesting that this region plays a role in iron acquisition that is shared by both TbpA and TbpB.
Figures

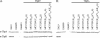


Similar articles
-
Evidence of Fe3+ interaction with the plug domain of the outer membrane transferrin receptor protein of Neisseria gonorrhoeae: implications for Fe transport.Metallomics. 2012 Apr;4(4):361-72. doi: 10.1039/c2mt20037f. Epub 2012 Mar 8. Metallomics. 2012. PMID: 22399131 Free PMC article.
-
Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB.J Bacteriol. 2002 Nov;184(22):6138-45. doi: 10.1128/JB.184.22.6138-6145.2002. J Bacteriol. 2002. PMID: 12399483 Free PMC article.
-
Point Mutations in TbpA Abrogate Human Transferrin Binding in Neisseria gonorrhoeae.Infect Immun. 2022 Nov 17;90(11):e0041422. doi: 10.1128/iai.00414-22. Epub 2022 Nov 2. Infect Immun. 2022. PMID: 36321833 Free PMC article.
-
Iron acquisition through the bacterial transferrin receptor.Crit Rev Biochem Mol Biol. 2017 Jun;52(3):314-326. doi: 10.1080/10409238.2017.1293606. Epub 2017 Mar 1. Crit Rev Biochem Mol Biol. 2017. PMID: 28276700 Review.
-
Transferrin-iron uptake by Gram-negative bacteria.Front Biosci. 2003 May 1;8:d836-47. doi: 10.2741/1076. Front Biosci. 2003. PMID: 12700102 Review.
Cited by
-
Patterns of structural and sequence variation within isotype lineages of the Neisseria meningitidis transferrin receptor system.Microbiologyopen. 2015 Jun;4(3):491-504. doi: 10.1002/mbo3.254. Epub 2015 Mar 19. Microbiologyopen. 2015. PMID: 25800619 Free PMC article.
-
Investigating the importance of selected surface-exposed loops in HpuB for hemoglobin binding and utilization by Neisseria gonorrhoeae.Infect Immun. 2024 Jul 11;92(7):e0021124. doi: 10.1128/iai.00211-24. Epub 2024 Jun 12. Infect Immun. 2024. PMID: 38864605 Free PMC article.
-
Protection of mice from a Chlamydia trachomatis vaginal infection using a Salicylidene acylhydrazide, a potential microbicide.J Infect Dis. 2011 Nov;204(9):1313-20. doi: 10.1093/infdis/jir552. Epub 2011 Sep 20. J Infect Dis. 2011. PMID: 21933873 Free PMC article.
-
Structural Basis for Evasion of Nutritional Immunity by the Pathogenic Neisseriae.Front Microbiol. 2020 Jan 10;10:2981. doi: 10.3389/fmicb.2019.02981. eCollection 2019. Front Microbiol. 2020. PMID: 31998268 Free PMC article. Review.
-
TonB-Dependent Transporters Expressed by Neisseria gonorrhoeae.Front Microbiol. 2011 May 27;2:117. doi: 10.3389/fmicb.2011.00117. eCollection 2011. Front Microbiol. 2011. PMID: 21747812 Free PMC article.
References
-
- Armstrong, S. K., and M. A. McIntosh. 1995. Epitope insertions define functional and topological features of the Escherichia coli ferric enterobactin receptor. J. Biol. Chem. 2702483-2488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

