T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa
- PMID: 18347048
- PMCID: PMC2346712
- DOI: 10.1128/IAI.01691-07
T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa
Abstract
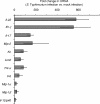
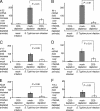
Salmonella enterica serotype Typhimurium causes an acute inflammatory reaction in the ceca of streptomycin-pretreated mice. We determined global changes in gene expression elicited by serotype Typhimurium in the cecal mucosa. The gene expression profile was dominated by T-cell-derived cytokines and genes whose expression is known to be induced by these cytokines. Markedly increased mRNA levels of genes encoding gamma interferon (IFN-gamma), interleukin-22 (IL-22), and IL-17 were detected by quantitative real-time PCR. Furthermore, the mRNA levels of genes whose expression is induced by IFN-gamma, IL-22, or IL-17, including genes encoding macrophage inflammatory protein 2 (MIP-2), inducible nitric oxide synthase (Nos2), lipocalin-2 (Lcn2), MIP-1alpha, MIP-1beta, and keratinocyte-derived cytokine (KC), were also markedly increased. To assess the importance of T cells in orchestrating this proinflammatory gene expression profile, we depleted T cells by using a monoclonal antibody prior to investigating cecal inflammation caused by serotype Typhimurium in streptomycin-pretreated mice. Depletion of CD3+ T cells resulted in a dramatic reduction in gross pathology, a significantly reduced recruitment of neutrophils, and a marked reduction in mRNA levels of Ifn-gamma, Il-22, Il-17, Nos2, Lcn2, and Kc. Our results suggest that T cells play an important role in amplifying inflammatory responses induced by serotype Typhimurium in the cecal mucosa.
Figures








References
-
- Barthel, M., S. Hapfelmeier, L. Quintanilla-Martínez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W.-D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. - PMC - PubMed
-
- Chen, L. M., S. Hobbie, and J. E. Galán. 1996. Requirement of cdc42 for salmonella-induced cytoskeletal and nuclear responses. Science 2742115-2118. - PubMed
-
- Day, D. W., B. K. Mandal, and B. C. Morson. 1978. The rectal biopsy appearances in Salmonella colitis. Histopathology 2117-131. - PubMed
-
- D'Orazio, S. E., M. J. Troese, and M. N. Starnbach. 2006. Cytosolic localization of Listeria monocytogenes triggers an early IFN-gamma response by CD8+ T cells that correlates with innate resistance to infection. J. Immunol. 1777146-7154. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

