Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1
- PMID: 18347054
- PMCID: PMC2423154
- DOI: 10.1128/MCB.01791-07
Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1
Abstract
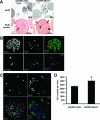
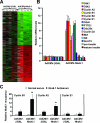
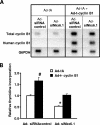
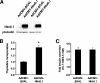
The homeodomain transcription factor Nkx6.1 plays an important role in pancreatic islet beta-cell development, but its effects on adult beta-cell function, survival, and proliferation are not well understood. In the present study, we demonstrated that treatment of primary rat pancreatic islets with a cytomegalovirus promoter-driven recombinant adenovirus containing the Nkx6.1 cDNA (AdCMV-Nkx6.1) causes dramatic increases in [methyl-(3)H] thymidine and 5-bromo-2'-deoxyuridine (BrdU) incorporation and in the number of cells per islet relative to islets treated with a control adenovirus (AdCMV-betaGAL), whereas suppression of Nkx6.1 expression reduces thymidine incorporation. Immunocytochemical studies reveal that >80% of BrdU-positive cells in AdCMV-Nkx6.1-treated islets are beta cells. Microarray, real-time PCR, and immunoblot analyses reveal that overexpression of Nkx6.1 in rat islets causes concerted upregulation of a cadre of cell cycle control genes, including those encoding cyclins A, B, and E, and several regulatory kinases. Cyclin E is upregulated earlier than the other cyclins, and adenovirus-mediated overexpression of cyclin E is shown to be sufficient to activate islet cell proliferation. Moreover, chromatin immunoprecipitation assays demonstrate direct interaction of Nkx6.1 with the cyclin A2 and B1 genes. Overexpression of Nkx6.1 in rat islets caused a clear enhancement of glucose-stimulated insulin secretion (GSIS), whereas overexpression of Nkx6.1 in human islets caused an increase in the level of [(3)H]thymidine incorporation that was twice the control level, along with complete retention of GSIS. We conclude that Nkx6.1 is among the very rare factors capable of stimulating beta-cell replication with retention or enhancement of function, properties that may be exploitable for expansion of beta-cell mass in treatment of both major forms of diabetes.
Figures



References
-
- Aleem, E., H. Kiyokawa, and P. Kaldis. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7831-836. - PubMed
-
- Bain, J. R., J. C. Schisler, K. Takeuchi, C. B. Newgard, and T. C. Becker. 2004. An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of langerhans. Diabetes 532190-2194. - PubMed
-
- Beattie, G. M., A. M. Montgomery, A. D. Lopez, E. Hao, B. Perez, M. L. Just, J. R. Lakey, M. E. Hart, and A. Hayek. 2002. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes 513435-3439. - PubMed
-
- Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43A161-189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
