Auxin influx carriers stabilize phyllotactic patterning
- PMID: 18347099
- PMCID: PMC2275433
- DOI: 10.1101/gad.462608
Auxin influx carriers stabilize phyllotactic patterning
Abstract
One of the most striking features of plant architecture is the regular arrangement of leaves and flowers around the stem, known as phyllotaxis. Peaks in concentration of the plant hormone auxin, generated by the polar localization of the PIN1 auxin efflux carrier, provide the instructive signal for primordium initiation. This mechanism generates the spacing between neighboring primordia, which results in regular phyllotaxis. Studies of the role of auxin transport in phyllotactic patterning have focused on PIN1-mediated efflux. Recent computer simulations indicate an additional role for transporter-mediated auxin uptake. Mutations in the AUX1 auxin influx carrier have not, however, been reported to cause an aerial phenotype. Here, we study the role of AUX1 and its paralogs LAX1, LAX2, and LAX3. Analysis of the quadruple mutant reveals irregular divergence angles between successive primordia. A highly unusual aspect of the phenotype is the occurrence of clusters of primordia, in violation of classical theory. At the molecular level, the sharp peaks in auxin levels and coordinated PIN polarization are reduced or lost. In addition, the increased penetrance of the phenotype under short-day conditions suggests that the AUX LAX transporters act to buffer the PIN-mediated patterning mechanism against environmental or developmental influences.
Figures


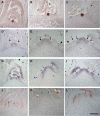

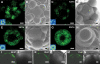


References
-
- Abas L., Benjamins R., Malenica N., Paciorek T., Wirniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006;8:249–256. - PubMed
-
- Aida M., Vernoux T., Furutani M., Traas J., Tasaka M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development. 2002;129:3965–3974. - PubMed
-
- Benkova E., Michniewicz M., Sauer M., Teichmann T., Jurgens G., Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. - PubMed
-
- Bennett M.J., Marchant A., Green H.G., May S.T., Ward S.P., Millner P.A., Walker A.R., Schulz B., Feldmann K.A. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science. 1996;273:948–950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
