Inhibition of androgen-independent prostate cancer cell growth is enhanced by combination therapy targeting Hedgehog and ErbB signalling
- PMID: 18348720
- PMCID: PMC2311276
- DOI: 10.1186/1475-2867-8-3
Inhibition of androgen-independent prostate cancer cell growth is enhanced by combination therapy targeting Hedgehog and ErbB signalling
Abstract
Background: Prostate cancer is a leading cause of male cancer specific mortality. When cure by radical prostatectomy is not possible the next line of prostate cancer treatment is androgen deprivation. However prolonged androgen deprivation often results in relapse and androgen-independent prostate cancer that is inevitably fatal despite optimal chemotherapy. The Hedgehog signalling pathway has recently been implicated in prostate cancer development and metastasis. EGFR or ErbB2 expression has been also correlated with androgen independence, shorter survival and metastasis.
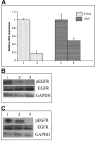
Results: We determined that the Hedgehog and ErbB signalling pathways are active in circulating tumour cells isolated from androgen-independent prostate cancer patients and in the androgen-independent prostate cancer cell line LNCaP C4-2B. As a basis for synergistic chemotherapy protocols combinations of the Hedgehog specific inhibitor cyclopamine and the ErbB signalling inhibitors gefitinib or lapatinib were tested in this study. Androgen-independent prostate cancer cell growth was inhibited by a SMO inhibitor (cyclopamine) which blocks Hedgehog signalling and by ErbB inhibitors (gefitinib and lapatinib). The isobologram and combination index method of Chou and Talalay was used to evaluate drug interactions. Synergistic antiproliferation effects were observed when the Hedgehog and ErbB inhibitors were combined.
Conclusion: Androgen-independent prostate cancer cell proliferation was associated with activity of the Hedgehog and ErbB signalling pathways. Cyclopamine, gefitinib or lapatinib treatment significantly decreased the proliferation of androgen-independent prostate cancer cells. The Hedgehog pathway therefore represents a promising new therapeutic target in androgen-independent prostate cancer. Synergistic effects were observed when Hedgehog and ErbB inhibitors were used together. This study may have clinical implications for improving the treatment of advanced prostate cancer.
Figures




Similar articles
-
Hedgehog signalling in androgen independent prostate cancer.Eur Urol. 2008 Dec;54(6):1333-43. doi: 10.1016/j.eururo.2008.01.070. Epub 2008 Feb 4. Eur Urol. 2008. PMID: 18262716
-
Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells.Mol Cancer Ther. 2007 Mar;6(3):967-78. doi: 10.1158/1535-7163.MCT-06-0648. Mol Cancer Ther. 2007. PMID: 17363490
-
Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells.Int J Cancer. 2006 Feb 15;118(4):1022-31. doi: 10.1002/ijc.21440. Int J Cancer. 2006. PMID: 16108016
-
Involvement of growth factor receptors of the epidermal growth factor receptor family in prostate cancer development and progression to androgen independence.Clin Prostate Cancer. 2003 Jun;2(1):50-7. doi: 10.3816/cgc.2003.n.013. Clin Prostate Cancer. 2003. PMID: 15046685 Review.
-
Hedgehog signaling in the prostate.J Urol. 2007 Mar;177(3):832-8. doi: 10.1016/j.juro.2006.10.061. J Urol. 2007. PMID: 17296352 Review.
Cited by
-
Hedgehog ligand and receptor cooperatively regulate EGFR stability and activity in non-small cell lung cancer.Cell Oncol (Dordr). 2024 Aug;47(4):1405-1423. doi: 10.1007/s13402-024-00938-6. Epub 2024 Apr 3. Cell Oncol (Dordr). 2024. PMID: 38568419
-
Looping forward: exploring R-loop processing and therapeutic potential.FEBS Lett. 2025 Jan;599(2):244-266. doi: 10.1002/1873-3468.14947. Epub 2024 Jun 6. FEBS Lett. 2025. PMID: 38844597 Free PMC article. Review.
-
Combined effects of cisplatin and photon or proton irradiation in cultured cells: radiosensitization, patterns of cell death and cell cycle distribution.J Radiat Res. 2020 Nov 16;61(6):832-841. doi: 10.1093/jrr/rraa065. J Radiat Res. 2020. PMID: 32880637 Free PMC article.
-
Network and data integration for biomarker signature discovery via network smoothed T-statistics.PLoS One. 2013 Sep 3;8(9):e73074. doi: 10.1371/journal.pone.0073074. eCollection 2013. PLoS One. 2013. PMID: 24019896 Free PMC article.
-
Inhibition of hedgehog signaling improves the anti-carcinogenic effects of docetaxel in prostate cancer.Oncotarget. 2015 Feb 28;6(6):3887-903. doi: 10.18632/oncotarget.2932. Oncotarget. 2015. PMID: 25682877 Free PMC article.
References
-
- Carson CC., 3rd Carcinoma of the prostate: overview of the most common malignancy in men. N C Med J. 2006;67:122–127. - PubMed
-
- Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo. 1994;8:439–443. - PubMed
-
- Shaw GL, Wilson P, Cuzick J, Prowse DM, Goldenberg SL, Spry NA, Oliver T. International study into the use of intermittent hormone therapy in the treatment of carcinoma of the prostate: a meta-analysis of 1446 patients. BJU Int. 2007;99:1056–1065. doi: 10.1111/j.1464-410X.2007.06770.x. - DOI - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

