Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis
- PMID: 18348981
- PMCID: PMC2386934
- DOI: 10.1074/jbc.M707733200
Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis
Abstract
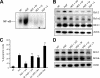
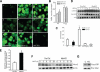
The hepatocyte growth factor and its receptor c-Met direct a pleiotropic signal transduction pathway that controls cell survival. We previously demonstrated that mice lacking c-Met (Met-KO) in hepatocytes were hypersensitive to Fas-induced liver injury. In this study, we used primary hepatocytes isolated from Met-KO and control (Cre-Ctrl) mice to address more directly the protective effects of c-Met signaling. Loss of c-Met function increased sensitivity to Fas-mediated apoptosis. Hepatocyte growth factor suppressed apoptosis in Cre-Ctrl but not Met-KO hepatocytes concurrently with up-regulation of NF-kappaB and major antiapoptotic proteins Bcl-2 and Bcl-xL. Intriguingly, Met-KO hepatocytes exhibited intrinsic activation of NF-kappaBas well as Bcl-2 and Bcl-xL. Furthermore, unchallenged Met-KO cells displayed oxidative stress as evidenced by overproduction of reactive oxygen species, which was associated with greater NADPH and Rac1 activities, was blocked by the known NADPH oxidase inhibitors, and was paralleled by increased lipid peroxidation and reduced glutathione (GSH) content. N-Acetylcysteine, an antioxidant and GSH precursor, significantly reduced Jo2-induced cell death. Conversely, the GSH-depleting agent buthionine sulfoximine completely abolished the protective effects of N-acetylcysteine in Met-KO hepatocytes. In conclusion, genetic inactivation of c-Met in mouse hepatocytes caused defects in redox regulation, which may account for the increased sensitivity to Fas-induced apoptosis and adaptive up-regulation of NF-kappaB survival signaling. These data provide evidence that intact c-Met signaling is a critical factor in the protection against excessive generation of endogenous reactive oxygen species.
Figures


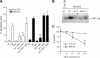
Similar articles
-
Adult Hepatocytes Are Hedgehog-Responsive Cells in the Setting of Liver Injury: Evidence for Smoothened-Mediated Activation of NF-κB/Epidermal Growth Factor Receptor/Akt in Hepatocytes that Counteract Fas-Induced Apoptosis.Am J Pathol. 2018 Nov;188(11):2605-2616. doi: 10.1016/j.ajpath.2018.07.018. Am J Pathol. 2018. PMID: 30366594 Free PMC article.
-
Akt protects mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis through NK-kappa B activation.Am J Physiol Gastrointest Liver Physiol. 2001 Dec;281(6):G1357-68. doi: 10.1152/ajpgi.2001.281.6.G1357. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11705740
-
miR-150 Deficiency Protects against FAS-Induced Acute Liver Injury in Mice through Regulation of AKT.PLoS One. 2015 Jul 21;10(7):e0132734. doi: 10.1371/journal.pone.0132734. eCollection 2015. PLoS One. 2015. PMID: 26196694 Free PMC article.
-
Tumor necrosis factor signaling in hepatocyte apoptosis.J Gastroenterol Hepatol. 2007 Jun;22 Suppl 1:S43-4. doi: 10.1111/j.1440-1746.2006.04645.x. J Gastroenterol Hepatol. 2007. PMID: 17567463 Review.
-
Redox regulation of hepatocyte apoptosis.J Gastroenterol Hepatol. 2007 Jun;22 Suppl 1:S38-42. doi: 10.1111/j.1440-1746.2006.04644.x. J Gastroenterol Hepatol. 2007. PMID: 17567462 Review.
Cited by
-
Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation.Hepatology. 2016 Nov;64(5):1711-1724. doi: 10.1002/hep.28721. Epub 2016 Oct 1. Hepatology. 2016. PMID: 27397846 Free PMC article.
-
t-BHQ Protects Against Oxidative Damage and Maintains the Antioxidant Response in Malnourished Rats.Dose Response. 2018 Sep 25;16(3):1559325818796304. doi: 10.1177/1559325818796304. eCollection 2018 Jul-Sep. Dose Response. 2018. PMID: 30263018 Free PMC article.
-
Protection against Fas-induced fulminant hepatic failure in liver specific integrin linked kinase knockout mice.Comp Hepatol. 2011 Nov 21;10:11. doi: 10.1186/1476-5926-10-11. Comp Hepatol. 2011. PMID: 22104495 Free PMC article.
-
Tissue injury alters the site of expression of hepatocyte growth factor activator inhibitor type 1 in bronchial epithelial cells.Hum Cell. 2009 Feb;22(1):11-7. doi: 10.1111/j.1749-0774.2008.00062.x. Hum Cell. 2009. PMID: 19222607
-
TRIB1 regulates liver regeneration by antagonizing the NRF2-mediated antioxidant response.Cell Death Dis. 2023 Jun 24;14(6):372. doi: 10.1038/s41419-023-05896-9. Cell Death Dis. 2023. PMID: 37355685 Free PMC article.
References
-
- Peter, M. E., and Krammer, P. H. (2003) Cell Death Differ. 10 26-35 - PubMed
-
- Ogasawara, J., Watanabe-Fukunaga, R., Adachi, M., Matsuzawa, A., Kasugai, T., Kitamura, Y., Itoh, N., Suda, T., and Nagata, S. (1993) Nature 364 806-809 - PubMed
-
- Gao, C. F., and Vande Woude, G. F. (2005) Cell Res. 15 49-51 - PubMed
-
- Comoglio, P. M. (2001) Nat. Cell Biol. 3 E161-E162 - PubMed
-
- Matsumoto, K., and Nakamura, T. (1996) J. Biochem. (Tokyo) 119 591-600 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

