Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience
- PMID: 18349817
- PMCID: PMC2275488
- DOI: 10.1038/sj.bjc.6604218
Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience
Abstract
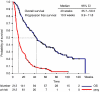
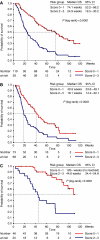
The main aim of phase I trials is to evaluate the tolerability and pharmacology of a new compound. However, investigating the potential for clinical benefit is also a key objective. Our phase I trial portfolio incorporates a range of new drugs, including molecular targeted agents, sometimes given together with cytotoxic agents. We performed this analysis of response rate, progression-free (PFS) and overall survival (OS) to assess the extent of clinical benefit rate (CBR: partial response (PR)+stable disease (SD)) derived from current trials. We analysed 212 consecutive patients who were treated in 29 phase I studies, from January 2005 to June 2006. All patients had progression of disease prior to study entry. The median age was 58 years (range: 18-86) with a male/female ratio of 2 : 1. A total of 148 patients (70%) were treated in 'first in human trials' involving biological agents (132 patients) or new cytotoxic compounds (16 patients) alone, and 64 patients (30%) received chemotherapy-based regimens with or without biological agents. After a median follow-up time of 34 weeks, the median PFS and OS were 11 and 43 weeks, respectively. The CBR was 53% (9% PR and 44% SD) after the first tumour evaluation after two cycles (between weeks 6 and 8) and has been maintained at 36 and 26% at 3 and 6 months, respectively. Treatment related deaths occurred in 0.47% of our patients and treatment had to be withdrawn in 11.8% of patients due to toxicity. A multivariate analysis (MVA) of 13 factors indicated that low albumin (<35 g l(-1)), lactate dehydrogenase>upper normal limit and >2 sites of metastasis were independent negative prognostic factors for OS. A risk score based on the MVA revealed that patients with a score of 2-3 had a significantly shorter OS compared to patients with a score of 0-1 (24.9 weeks, 95% CI 19.5-30.2 vs 74.1 weeks, 95% CI 53.2-96.2). This analysis shows that a significant number of patients who develop disease progression while receiving standard therapy derived benefit from participation in phase I trials. Risk scoring based on objective clinical parameters indicated that patients with a high score had a significantly shorter OS, and this may help in the process of patient selection for phase I trial entry.
Figures
Comment in
-
Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience.Br J Cancer. 2008 Oct 21;99(8):1364; author reply 1365. doi: 10.1038/sj.bjc.6604648. Epub 2008 Sep 9. Br J Cancer. 2008. PMID: 18781182 Free PMC article. No abstract available.
References
-
- Bachelot T, Ray-Coquard I, Catimel G, Ardiet C, Guastalla JP, Dumortier A, Chauvin F, Droz JP, Philip T, Clavel M (2000) Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase-I clinical trials. Ann Oncol 11: 151–156 - PubMed
-
- Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Vollmer R, Wilding G (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 17: 3461–3467 - PubMed
-
- Cox DR (1972) Regression models and life tables. J Roy Stat Soc (B) 34: 187–202
-
- Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, Shoemaker D, Emanuel EJ, Grady C (2005) Risks and benefits of phase-1 oncology trials, 1991 through 2002. N Engl J Med 352: 895–904 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

