Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators
- PMID: 18350138
- PMCID: PMC2262942
- DOI: 10.1371/journal.pone.0001796
Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators
Abstract
Genetically-encoded calcium indicators (GECIs) hold the promise of monitoring [Ca(2+)] in selected populations of neurons and in specific cellular compartments. Relating GECI fluorescence to neuronal activity requires quantitative characterization. We have characterized a promising new genetically-encoded calcium indicator-GCaMP2-in mammalian pyramidal neurons. Fluorescence changes in response to single action potentials (17+/-10% DeltaF/F [mean+/-SD]) could be detected in some, but not all, neurons. Trains of high-frequency action potentials yielded robust responses (302+/-50% for trains of 40 action potentials at 83 Hz). Responses were similar in acute brain slices from in utero electroporated mice, indicating that long-term expression did not interfere with GCaMP2 function. Membrane-targeted versions of GCaMP2 did not yield larger signals than their non-targeted counterparts. We further targeted GCaMP2 to dendritic spines to monitor Ca(2+) accumulations evoked by activation of synaptic NMDA receptors. We observed robust DeltaF/F responses (range: 37%-264%) to single spine uncaging stimuli that were correlated with NMDA receptor currents measured through a somatic patch pipette. One major drawback of GCaMP2 was its low baseline fluorescence. Our results show that GCaMP2 is improved from the previous versions of GCaMP and may be suited to detect bursts of high-frequency action potentials and synaptic currents in vivo.
Conflict of interest statement
Figures

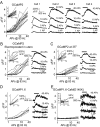

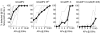
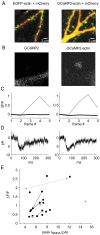
References
-
- Regehr WG, Connor JA, Tank DW. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature. 1989;341:533–536. - PubMed
-
- Tank DW, Sugimori M, Connor JA, Llinas RR. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988;242:773–777. - PubMed
-
- Jaffe DB, Johnston D, Lasser RN, Lisman JE, Miyakawa H, et al. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992;357:244–246. - PubMed
-
- Callaway JC, Ross WN. Frequency-dependent propagation of sodium action potentials in dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1995;74:1395–1403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

