Measuring melatonin in humans
- PMID: 18350967
- PMCID: PMC2276833
Measuring melatonin in humans
Abstract
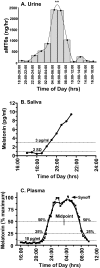
Study objectives: To provide guidelines for collecting and analyzing urinary, salivary, and plasma melatonin, thereby assisting clinicians and researchers in determining which method of measuring melatonin is most appropriate for their particular needs and facilitating the comparison of data between laboratories.
Methods: A modified RAND process was utilized to derive recommendations for methods of measuring melatonin in humans.
Results: Consensus-based guidelines are presented for collecting and analyzing melatonin for studies that are conducted in the natural living environment, the clinical setting, and in-patient research facilities under controlled conditions.
Conclusions: The benefits and disadvantages of current methods of collecting and analyzing melatonin are summarized. Although a single method of analysis would be the most effective way to compare studies, limitations of current methods preclude this possibility. Given that the best analysis method for use under multiple conditions is not established, it is recommended to include, in any published report, one of the established low threshold measures of dim light melatonin onset to facilitate comparison between studies.
Figures
References
-
- Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms. 2005;20:291–303. - PubMed
-
- Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Balériaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. - PubMed
-
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. - PubMed
-
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. - PubMed
-
- Fitch K, Berstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica: RAND Corporation; 2001.
Publication types
MeSH terms
Substances
Grants and funding
- HL072408/HL/NHLBI NIH HHS/United States
- R01 MH063462/MH/NIMH NIH HHS/United States
- K02 HD045459/HD/NICHD NIH HHS/United States
- MH-59919/MH/NIMH NIH HHS/United States
- R01 HL072408/HL/NHLBI NIH HHS/United States
- NR007677/NR/NINR NIH HHS/United States
- R01 AG012112/AG/NIA NIH HHS/United States
- R01 HL086934/HL/NHLBI NIH HHS/United States
- MH-063462/MH/NIMH NIH HHS/United States
- P01 AG009975/AG/NIA NIH HHS/United States
- MH-070788/MH/NIMH NIH HHS/United States
- HL086934/HL/NHLBI NIH HHS/United States
- R01 MH059919/MH/NIMH NIH HHS/United States
- R56 AG15370/AG/NIA NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- M01-RR-00827/RR/NCRR NIH HHS/United States
- HL 67604/HL/NHLBI NIH HHS/United States
- R56 AG015370/AG/NIA NIH HHS/United States
- K02-HD045459/HD/NICHD NIH HHS/United States
- R01 NR007677/NR/NINR NIH HHS/United States
- R01 HL067604/HL/NHLBI NIH HHS/United States
- R01 MH070788/MH/NIMH NIH HHS/United States
- R01 AG12112/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
