Active and passive immunotherapy for neurodegenerative disorders
- PMID: 18352830
- PMCID: PMC2561172
- DOI: 10.1146/annurev.neuro.31.060407.125529
Active and passive immunotherapy for neurodegenerative disorders
Abstract
Immunotherapeutic strategies to combat neurodegenerative disorders have galvanized the scientific community since the first dramatic successes in mouse models recreating aspects of Alzheimer disease (AD) were reported. However, initial human trials of active amyloid-beta (Abeta) vaccination were halted early because of a serious safety issue: meningoencephalitis in 6% of subjects. Nonetheless, some encouraging preliminary data were obtained, and rapid progress has been made toward developing alternative, possibly safer active and passive immunotherapeutic approaches for several neurodegenerative conditions. Many of these are currently in human trials for AD. Despite these advances, our understanding of the essential mechanisms underlying the effects seen in preclinical models and human subjects is still incomplete. Antibody-induced phagocytosis of pathological protein deposits, direct antibody-mediated disruption of aggregates, neutralization of toxic soluble proteins, a shift in equilibrium toward efflux of specific proteins from the brain, cell-mediated immune responses, and other mechanisms may all play roles depending on the specific immunotherapeutic scenario.
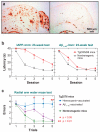
Figures


References
-
- Austin L, Arendash GW, Gordon MN, Diamond DM, DiCarlo G, et al. Short-term beta-amyloid vaccinations do not improve cognitive performance in cognitively impaired APP + PS1 mice. Behav. Neurosci. 2003;117:478–84. - PubMed
-
- Bade S, Frey A. Potential of active and passive immunizations for the prevention and therapy of transmissible spongiform encephalopathies. Expert Rev. Vaccin. 2007;6:153–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

