Role of the conserved oligomeric Golgi (COG) complex in protein glycosylation
- PMID: 18353293
- PMCID: PMC2773262
- DOI: 10.1016/j.carres.2008.01.034
Role of the conserved oligomeric Golgi (COG) complex in protein glycosylation
Abstract
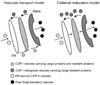
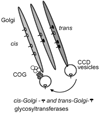
The Golgi apparatus is a central hub for both protein and lipid trafficking/sorting and is also a major site for glycosylation in the cell. This organelle employs a cohort of peripheral membrane proteins and protein complexes to keep its structural and functional organization. The conserved oligomeric Golgi (COG) complex is an evolutionary conserved peripheral membrane protein complex that is proposed to act as a retrograde vesicle tethering factor in intra-Golgi trafficking. The COG protein complex consists of eight subunits, distributed in two lobes, Lobe A (Cog1-4) and Lobe B (Cog5-8). Malfunctions in the COG complex have a significant impact on processes such as protein sorting, glycosylation, and Golgi integrity. A deletion of Lobe A COG subunits in yeasts causes severe growth defects while mutations in COG1, COG7, and COG8 in humans cause novel types of congenital disorders of glycosylation. These pathologies involve a change in structural Golgi phenotype and function. Recent results indicate that down-regulation of COG function results in the resident Golgi glycosyltransferases/glycosidases to be mislocalized or degraded.
Figures

Similar articles
-
Genetic analysis of the subunit organization and function of the conserved oligomeric golgi (COG) complex: studies of COG5- and COG7-deficient mammalian cells.J Biol Chem. 2005 Sep 23;280(38):32736-45. doi: 10.1074/jbc.M505558200. Epub 2005 Jul 28. J Biol Chem. 2005. PMID: 16051600
-
Deficiency of the Cog8 subunit in normal and CDG-derived cells impairs the assembly of the COG and Golgi SNARE complexes.Traffic. 2013 Oct;14(10):1065-77. doi: 10.1111/tra.12093. Epub 2013 Jul 31. Traffic. 2013. PMID: 23865579 Free PMC article.
-
Conserved Oligomeric Golgi and Neuronal Vesicular Trafficking.Handb Exp Pharmacol. 2018;245:227-247. doi: 10.1007/164_2017_65. Handb Exp Pharmacol. 2018. PMID: 29063274 Free PMC article. Review.
-
Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery.Glycobiology. 2011 Dec;21(12):1554-69. doi: 10.1093/glycob/cwr028. Epub 2011 Mar 18. Glycobiology. 2011. PMID: 21421995 Free PMC article.
-
Deficiencies in subunits of the Conserved Oligomeric Golgi (COG) complex define a novel group of Congenital Disorders of Glycosylation.Mol Genet Metab. 2008 Jan;93(1):15-21. doi: 10.1016/j.ymgme.2007.08.118. Epub 2007 Sep 29. Mol Genet Metab. 2008. PMID: 17904886 Review.
Cited by
-
Transcriptomic Signatures of the Foetal Liver and Late Prenatal Development in Vitrified Rabbit Embryos.Vet Sci. 2024 Aug 1;11(8):347. doi: 10.3390/vetsci11080347. Vet Sci. 2024. PMID: 39195801 Free PMC article.
-
A three-stage model of Golgi structure and function.Histochem Cell Biol. 2013 Sep;140(3):239-49. doi: 10.1007/s00418-013-1128-3. Epub 2013 Jul 24. Histochem Cell Biol. 2013. PMID: 23881164 Free PMC article. Review.
-
Tumor Targeting via siRNA-COG3 to Suppress Tumor Progression in Mice and Inhibit Cancer Metastasis and Angiogenesis in Ovarian Cancer Cell Lines.Microrna. 2024;13(2):140-154. doi: 10.2174/0122115366275856240101083442. Microrna. 2024. PMID: 38243930
-
Defective GM3 synthesis in Cog2 null mutant CHO cells associates to mislocalization of lactosylceramide sialyltransferase in the Golgi complex.Neurochem Res. 2010 Dec;35(12):2161-7. doi: 10.1007/s11064-010-0319-8. Epub 2010 Nov 17. Neurochem Res. 2010. PMID: 21080064
-
COG7 deficiency in Drosophila generates multifaceted developmental, behavioral and protein glycosylation phenotypes.J Cell Sci. 2017 Nov 1;130(21):3637-3649. doi: 10.1242/jcs.209049. Epub 2017 Sep 7. J Cell Sci. 2017. PMID: 28883096 Free PMC article.
References
-
- Warren G, Malhotra V. Curr. Opin. Cell Biol. 1998;10:493–498. - PubMed
-
- Bonifacino JS, Glick BS. Cell. 2004;116:153–166. - PubMed
-
- Shorter J, Warren G. Annu. Rev. Cell Dev. Biol. 2002;18:379–420. - PubMed
-
- Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T. J. Cell Sci. 1995;108:1617–1627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

