Allostery: absence of a change in shape does not imply that allostery is not at play
- PMID: 18353365
- PMCID: PMC2684958
- DOI: 10.1016/j.jmb.2008.02.034
Allostery: absence of a change in shape does not imply that allostery is not at play
Abstract
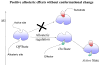
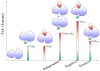
Allostery is essential for controlled catalysis, signal transmission, receptor trafficking, turning genes on and off, and apoptosis. It governs the organism's response to environmental and metabolic cues, dictating transient partner interactions in the cellular network. Textbooks taught us that allostery is a change of shape at one site on the protein surface brought about by ligand binding to another. For several years, it has been broadly accepted that the change of shape is not induced; rather, it is observed simply because a larger protein population presents it. Current data indicate that while side chains can reorient and rewire, allostery may not even involve a change of (backbone) shape. Assuming that the enthalpy change does not reverse the free-energy change due to the change in entropy, entropy is mainly responsible for binding.
Figures

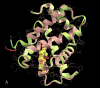
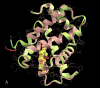
References
-
- Daily MD, Gray JJ. Local motions in a benchmark of allosteric proteins. Proteins Struct Funct Genet. 2007;67:385–399. - PubMed
-
- Swain JF, Gierasch LM. The changing landscape of protein allostery. Curr Opin Struct Biol. 2006;16:102–108. - PubMed
-
- Gunasekaran K, Ma BY, Nussinov R. Is allostery an intrinsic property of all dynamic proteins? Proteins Struct Funct Genet. 2004;57:433–443. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

