Dephosphorylation of JC virus agnoprotein by protein phosphatase 2A: inhibition by small t antigen
- PMID: 18353419
- PMCID: PMC2739041
- DOI: 10.1016/j.virol.2008.02.020
Dephosphorylation of JC virus agnoprotein by protein phosphatase 2A: inhibition by small t antigen
Abstract
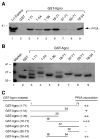
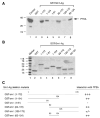
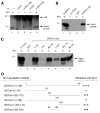
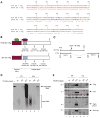
Previous studies have demonstrated that the JC virus (JCV) late regulatory protein agnoprotein is phosphorylated by the serine/threonine-specific protein kinase-C (PKC) and mutants of this protein at the PKC phosphorylation sites exhibit defects in the viral replication cycle. We have now investigated whether agnoprotein phosphorylation is regulated by PP2A, a serine/threonine-specific protein phosphatase and whether JCV small t antigen (Sm t-Ag) is involved in this regulation. Protein-protein interaction studies demonstrated that PP2A associates with agnoprotein and dephosphorylates it at PKC-specific sites. Sm t-Ag was also found to interact with PP2A and this interaction inhibited the dephosphorylation of agnoprotein by PP2A. The interaction domains of Sm t-Ag and agnoprotein with PP2A were mapped, as were the interaction domains of Sm t-Ag with agnoprotein. The middle portion of Sm t-Ag (aa 82-124) was found to be critical for the interaction with both agnoprotein and PP2A and the N-terminal region of agnoprotein for interaction with Sm t-Ag. To further understand the role of Sm t-Ag in JCV regulation, a stop codon was introduced at Ser90 immediately after splice donor site of the JCV early gene and the functional consequences of this mutation were investigated. The ability of this mutant virus to replicate was substantially reduced compared to WT. Next, the functional significance of PP2A in JCV replication was examined by siRNA targeting. Downregulation of PP2A caused a significant reduction in the level of JCV replication. Moreover, the impact of Sm t-Ag on agnoprotein phosphorylation was investigated by creating a double mutant of JCV, where Sm t-Ag stop codon mutant was combined with an agnoprotein triple phosphorylation mutant (Ser7, Ser11 and Thr21 to Ala). Results showed that double mutant behaves much like the triple phosphorylation mutant of agnoprotein during viral replication cycle, which suggests that agnoprotein might be an important target of Sm t-Ag with respect to the regulation of its phosphorylation. Collectively, these results suggest that there is an interplay between agnoprotein, Sm t-Ag and PP2A with respect to the regulation of JCV life cycle and this could be important for the progression of the JCV-induced disease, PML.
Figures




Similar articles
-
Phosphorylation mutants of JC virus agnoprotein are unable to sustain the viral infection cycle.J Virol. 2006 Apr;80(8):3893-903. doi: 10.1128/JVI.80.8.3893-3903.2006. J Virol. 2006. PMID: 16571806 Free PMC article.
-
Nuclear magnetic resonance structure revealed that the human polyomavirus JC virus agnoprotein contains an α-helix encompassing the Leu/Ile/Phe-rich domain.J Virol. 2014 Jun;88(12):6556-75. doi: 10.1128/JVI.00146-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672035 Free PMC article.
-
JC virus small T antigen binds phosphatase PP2A and Rb family proteins and is required for efficient viral DNA replication activity.PLoS One. 2010 May 12;5(5):e10606. doi: 10.1371/journal.pone.0010606. PLoS One. 2010. PMID: 20485545 Free PMC article.
-
The agnoprotein of polyomaviruses: a multifunctional auxiliary protein.J Cell Physiol. 2005 Jul;204(1):1-7. doi: 10.1002/jcp.20266. J Cell Physiol. 2005. PMID: 15573377 Review.
-
Emerging From the Unknown: Structural and Functional Features of Agnoprotein of Polyomaviruses.J Cell Physiol. 2016 Oct;231(10):2115-27. doi: 10.1002/jcp.25329. Epub 2016 Feb 24. J Cell Physiol. 2016. PMID: 26831433 Free PMC article. Review.
Cited by
-
Regulation of gene expression in primate polyomaviruses.J Virol. 2009 Nov;83(21):10846-56. doi: 10.1128/JVI.00542-09. Epub 2009 Jul 29. J Virol. 2009. PMID: 19640999 Free PMC article. Review.
-
Human polyomavirus modulation of the host DNA damage response.Virus Genes. 2020 Apr;56(2):128-135. doi: 10.1007/s11262-020-01736-6. Epub 2020 Jan 29. Virus Genes. 2020. PMID: 31997082 Review.
-
Regulation of human neurotropic JC virus replication by alternative splicing factor SF2/ASF in glial cells.PLoS One. 2011 Jan 31;6(1):e14630. doi: 10.1371/journal.pone.0014630. PLoS One. 2011. PMID: 21297941 Free PMC article.
-
Generation and characterization of JCV permissive hybrid cell lines.J Virol Methods. 2009 Jul;159(1):122-6. doi: 10.1016/j.jviromet.2009.02.023. Epub 2009 Mar 4. J Virol Methods. 2009. PMID: 19442856 Free PMC article.
-
The Role of the JC Virus in Central Nervous System Tumorigenesis.Int J Mol Sci. 2020 Aug 28;21(17):6236. doi: 10.3390/ijms21176236. Int J Mol Sci. 2020. PMID: 32872288 Free PMC article. Review.
References
-
- Akan I, Sariyer IK, Biffi R, Palermo V, Woolridge S, White MK, Amini S, Khalili K, Safak M. Human polyomavirus JCV late leader peptide region contains important regulatory elements. Virology. 2006;349(1):66–78. - PubMed
-
- Ali SH, DeCaprio JA. Cellular transformation by SV40 large Tantigen: interaction with host proteins. Semin Cancer Biol. 2001;11(1):15–23. - PubMed
-
- Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24(52):7746–7755. - PubMed
-
- Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirology. 1995;1(1):5–18. - PubMed
-
- Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer. 2007;7(7):495–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

