Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells
- PMID: 18353896
- PMCID: PMC3327159
- DOI: 10.1152/ajpcell.00096.2008
Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells
Abstract
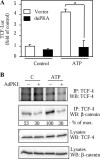
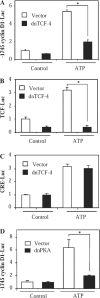
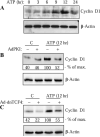
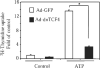
Extracellular ATP stimulates proliferation of vascular smooth muscle cells (VSMC) through activation of G protein-coupled P2Y purinergic receptors. We have previously shown that ATP stimulates a transient activation of protein kinase A (PKA), which, together with the established mitogenic signaling of purinergic receptors, promotes proliferation of VSMC (Hogarth DK, Sandbo N, Taurin S, Kolenko V, Miano JM, Dulin NO. Am J Physiol Cell Physiol 287: C449-C456, 2004). We also have shown that PKA can phosphorylate beta-catenin at two novel sites (Ser552 and Ser675) in vitro and in overexpression cell models (Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. J Biol Chem 281: 9971-9976, 2006). beta-Catenin promotes cell proliferation by activation of a family of T-cell factor (TCF) transcription factors, which drive the transcription of genes implicated in cell cycle progression including cyclin D1. In the present study, using the phosphospecific antibodies against phospho-Ser552 or phospho-Ser675 sites of beta-catenin, we show that ATP can stimulate PKA-dependent phosphorylation of endogenous beta-catenin at both of these sites without affecting its expression levels in VSMC. This translates to a PKA-dependent stimulation of TCF transcriptional activity through an increased association of phosphorylated (by PKA) beta-catenin with TCF-4. Using the PKA inhibitor PKI or dominant negative TCF-4 mutant, we show that ATP-induced cyclin D1 promoter activation, cyclin D1 protein expression, and proliferation of VSMC are all dependent on PKA and TCF activities. In conclusion, we show a novel mode of regulation of endogenous beta-catenin through its phosphorylation by PKA, and we demonstrate the importance of this mechanism for ATP-induced proliferation of VSMC.
Figures


References
-
- Abbott KL, Loss JR, 2nd, Robida AM, Murphy TJ. Evidence that Galpha(q)-coupled receptor-induced interleukin-6 mRNA in vascular smooth muscle cells involves the nuclear factor of activated T cells. Mol Pharmacol. 2000;58:946–953. - PubMed
-
- Abu-Amer Y, Ross FP, McHugh KP, Livolsi A, Peyron JF, Teitelbaum SL. Tumor necrosis factor-alpha activation of nuclear transcription factor-kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of Ikappa Balpha. J Biol Chem. 1998;273:29417–29423. - PubMed
-
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

