Kinesin motor domain of Leishmania donovani as a future vaccine candidate
- PMID: 18353921
- PMCID: PMC2394829
- DOI: 10.1128/CVI.00433-07
Kinesin motor domain of Leishmania donovani as a future vaccine candidate
Abstract
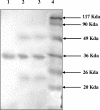
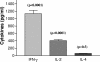
Visceral leishmaniasis (VL) is one of the important parasitic diseases, with approximately 350 million people at risk. Due to the nonavailability of an ideal drug, development of a safe, effective, and affordable vaccine could be a solution for control and prevention of this disease. The present study was carried out to examine the immunological potential of kinesin protein from the microtubule locus of Leishmania donovani as a suitable vaccine candidate. In silico analysis of this region revealed clusters of major histocompatibility complex class I and II binding epitopes in its motor domain region. A recombinant protein was expressed from this region and named rLvacc. The antigenicity and immunogenicity studies of this protein by Western blot analysis revealed that rLvacc is strongly recognized by sera from acute VL patients. To evaluate its immunogenicity, peripheral blood mononuclear cells from cured VL patients were separated, and a lymphocyte proliferation assay was carried out in the presence of rLvacc. After lymphocyte proliferation, the pooled culture supernatant was assayed for anti-rLvacc antibody titers using an enzyme-linked immunosorbent assay. The results showed that immunoglobulin G2 (IgG2) subtype antibodies were predominant, while IgG1 subtype antibodies were produced in very low titers. On the basis of these ex vivo preliminary findings, its immunogenicity was studied in BALB/c mice. Vaccination with the DNA construct generated a good cellular immune response with significant increases in gamma interferon and interleukin-2 (IL-2) cytokine levels (Th1), but no increase in IL-4 levels (Th2). Taken together, our findings suggest the kinesin motor domain region of L. donovani as a potential vaccine candidate against visceral leishmaniasis.
Figures

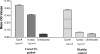



Similar articles
-
Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis.J Immunol. 2000 Dec 15;165(12):7064-71. doi: 10.4049/jimmunol.165.12.7064. J Immunol. 2000. PMID: 11120835
-
Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections.Vaccine. 2001 Oct 12;20(1-2):59-66. doi: 10.1016/s0264-410x(01)00322-x. Vaccine. 2001. PMID: 11567746
-
Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis.Infect Immun. 2001 Aug;69(8):4719-25. doi: 10.1128/IAI.69.8.4719-4725.2001. Infect Immun. 2001. PMID: 11447143 Free PMC article.
-
Leishmaniasis: current status of vaccine development.Curr Mol Med. 2004 Sep;4(6):667-79. doi: 10.2174/1566524043360203. Curr Mol Med. 2004. PMID: 15357215 Review.
-
Vaccines for visceral leishmaniasis: A review.J Immunol Methods. 2015 Jul;422:1-12. doi: 10.1016/j.jim.2015.03.017. Epub 2015 Apr 7. J Immunol Methods. 2015. PMID: 25858230 Review.
Cited by
-
Mutation Characteristics and Phylogenetic Analysis of Five Leishmania Clinical Isolates.Animals (Basel). 2022 Jan 28;12(3):321. doi: 10.3390/ani12030321. Animals (Basel). 2022. PMID: 35158645 Free PMC article.
-
Quantitative Proteome Analysis of Leishmania donovani under Spermidine Starvation.PLoS One. 2016 Apr 28;11(4):e0154262. doi: 10.1371/journal.pone.0154262. eCollection 2016. PLoS One. 2016. PMID: 27123864 Free PMC article.
-
HLA-DRB1 Alleles Associated with Lower Leishmaniasis Susceptibility Share Common Amino Acid Polymorphisms and Epitope Binding Repertoires.Vaccines (Basel). 2021 Mar 17;9(3):270. doi: 10.3390/vaccines9030270. Vaccines (Basel). 2021. PMID: 33803005 Free PMC article.
-
Evaluation of a rapid device for serological diagnosis of Leishmania infantum infection in dogs as an alternative to immunofluorescence assay and Western blotting.Clin Vaccine Immunol. 2013 May;20(5):657-9. doi: 10.1128/CVI.00719-12. Epub 2013 Feb 27. Clin Vaccine Immunol. 2013. PMID: 23446218 Free PMC article.
-
Possibilities and challenges for developing a successful vaccine for leishmaniasis.Parasit Vectors. 2016 May 12;9(1):277. doi: 10.1186/s13071-016-1553-y. Parasit Vectors. 2016. PMID: 27175732 Free PMC article. Review.
References
-
- Alarcon, J. B., G. W. Waine, and D. P. McManus. 1999. DNA vaccines: technology and application as anti-parasite and anti-microbial agents. Adv. Parasitol. 42:343-410. - PubMed
-
- Basu, R., S. Bhaumik, J. M. Basu, K. Naskar, T. De, and S. Roy. 2005. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J. Immunol. 174:7160-7171. - PubMed
-
- Burns, J. M., Jr., W. G. Shreffler, D. R. Benson, H. W. Ghalib, R. Badaro, and S. G. Reed. 1993. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral Leishmaniasis. Proc. Natl. Acad. Sci. USA 90:775-779. - PMC - PubMed
-
- Cenini, P., N. Berhe, A. Hailu, K. McGinnes, and D. Frommel. 1993. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J. Infect. Dis. 168:986-993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

