Infection of macrophages and dendritic cells with primary R5-tropic human immunodeficiency virus type 1 inhibited by natural polyreactive anti-CCR5 antibodies purified from cervicovaginal secretions
- PMID: 18353923
- PMCID: PMC2394833
- DOI: 10.1128/CVI.00463-07
Infection of macrophages and dendritic cells with primary R5-tropic human immunodeficiency virus type 1 inhibited by natural polyreactive anti-CCR5 antibodies purified from cervicovaginal secretions
Abstract
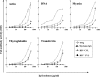
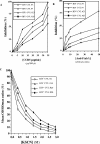
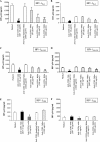
Heterosexual contact is the primary mode of human immunodeficiency virus (HIV) type 1 (HIV-1) transmission worldwide. The chemokine receptor CCR5 is the major coreceptor that is associated with the mucosal transmission of R5-tropic HIV-1 during sexual intercourse. The CCR5 molecule is thus a target for antibody-based therapeutic strategies aimed at blocking HIV-1 entry into cells. We have previously demonstrated that polyreactive natural antibodies (NAbs) from therapeutic preparations of immunoglobulin G and from human breast milk contain NAbs directed against CCR5. Such antibodies inhibit the infection of human macrophages and T lymphocytes by R5-tropic isolates of HIV in vitro. In the present study, we demonstrate that human immunoglobulins from the cervicovaginal secretions of HIV-seronegative or HIV-seropositive women contain NAbs directed against the HIV-1 coreceptor CCR5. Natural affinity-purified anti-CCR5 antibodies bound to CCR5 expressed on macrophages and dendritic cells and further inhibited the infection of macrophages and dendritic cells with primary and laboratory-adapted R5-tropic HIV but not with X4-tropic HIV. Natural anti-CCR5 antibodies moderately inhibited R5-tropic HIV transfer from monocyte-derived dendritic cells to autologous T cells. Our results suggest that mucosal anti-CCR5 antibodies from healthy immunocompetent donors may hamper the penetration of HIV and may be suitable for use in the development of novel passive immunotherapy regimens in specific clinical settings of HIV infection.
Figures




References
-
- Avrameas, S., G. Dighiero, P. Lymberi, and B. Guilbert. 1983. Studies on natural antibodies and autoantibodies. Ann. Immunol. (Paris) 134D:103-113. - PubMed
-
- Bard, E., D. Riethmuller, S. Biichle, D. Meillet, J. L. Pretet, C. Mougin, and E. Seilles. 2002. Validation of a high sensitive immunoenzymatic assay to establish the origin of immunoglobulins in female genital secretions. J. Immunoassay Immunochem. 23:145-162. - PubMed
-
- Baumgarth, N., J. W. Tung, and L. A. Herzenberg. 2005. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26:347-362. - PubMed
-
- Belec, L. 2002. Defenses of the female genital tract against infection. J. Gynecol. Obstet. Biol. Reprod (Paris) 31:4S45-4S59. (In French.) - PubMed
-
- Belec, L., T. Dupre, T. Prazuck, C. Tevi-Benissan, J. M. Kanga, O. Pathey, X. S. Lu, and J. Pillot. 1995. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J. Infect. Dis. 172:691-697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

