Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency
- PMID: 18353927
- PMCID: PMC6097533
- DOI: 10.1189/jlb.1207838
Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency
Abstract
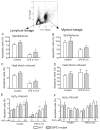
Bone marrow (BM) dysfunction is an important component of immunomodulation. This study investigated alterations in cell content, apoptotic responses, and cell proliferation in BM, blood, and spleen in endotoxemic mice (LPS from Escherichia coli). As the decreased antioxidant status associated with glucose-6-phosphate dehydrogenase (G6PD) deficiency has been shown to modulate the innate immune response, we also tested whether a G6PD mutation (80% decrease in cellular enzyme activity) alters BM responses during endotoxemia. LPS decreased BM myeloid (CD45(+)CD11b(+)) and B lymphoid (CD45(+)CD19(+)CD11b(-)) cell content compared with controls. In contrast, LPS increased CD11b(+) myeloid but decreased T and B cell counts in the circulation. Endotoxemia inhibited spontaneous, heat shock, and H(2)O(2)-induced apoptosis as well as proliferative activity in BM lymphoid cells. In contrast, BM myeloid cell apoptosis was not altered, and their proliferative activity was increased during endotoxemia. Following LPS, splenic myeloid cell content was increased, and T and B cell content was unchanged; furthermore, splenocytes showed increased apoptosis compared with controls. BM cell content, including lymphoid and myeloid cells, was greater in G6PD mutant than wild-type (WT) mice, and LPS decreased BM cell counts to a greater degree in mutant than WT mice. Endotoxemia caused widespread inhibition of BM cytokine and chemokine production; however, IL-6 production was increased compared with controls. LPS-induced IL-6 production was decreased in G6PD mutant animals compared with WT. This study indicates that endotoxin inversely affects BM myeloid and lymphoid cell production. LPS-induced down-regulation of B cell production contributes to the generalized lymphopenia and lymphocyte dysfunction observed following nonspecific immune challenges.
Figures



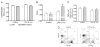


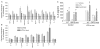
References
-
- Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. - PubMed
-
- Spolarics Z, Condon MR, Siddiqi M, Machiedo GW, Deitch EA. Red blood cell dysfunction in septic glucose-6-phosphate dehydrogenase-deficient mice. Am J Physiol Heart Circ Physiol. 2004;286:H2118–H2126. - PubMed
-
- Wilmanski J, Villanueva E, Deitch EA, Spolarics Z. Glucose-6-phosphate dehydrogenase deficiency and the inflammatory response to endotoxin and polymicrobial sepsis. Crit Care Med. 2007;35:510–518. - PubMed
-
- Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis—a potential treatment of sepsis? Clin Infect Dis. 2005;41(Suppl 7):S465–S469. - PubMed
-
- Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

