The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils
- PMID: 18353929
- PMCID: PMC2901559
- DOI: 10.1189/jlb.0907658
The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils
Abstract
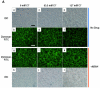
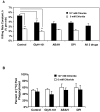
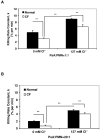
Chloride anion is essential for myeloperoxidase (MPO) to produce hypochlorous acid (HOCl) in polymorphonuclear neutrophils (PMNs). To define whether chloride availability to PMNs affects their HOCl production and microbicidal capacity, we examined how extracellular chloride concentration affects killing of Pseudomonas aeruginosa (PsA) by normal neutrophils. PMN-mediated bacterial killing was strongly dependent on extracellular chloride concentration. Neutrophils in a chloride-deficient medium killed PsA poorly. However, as the chloride level was raised, the killing efficiency increased in a dose-dependent manner. By using specific inhibitors to selectively block NADPH oxidase, MPO, and cystic fibrosis transmembrane conductance regulator (CFTR) functions, neutrophil-mediated killing of PsA could be attributed to three distinct mechanisms: CFTR-dependent and oxidant-dependent; chloride-dependent but not CFTR- and oxidant-dependent; and independent of any of the tested factors. Therefore, chloride anion is involved in oxidant- and nonoxidant-mediated bacterial killing. We previously reported that neutrophils from CF patients are defective in chlorination of ingested bacteria, suggesting that the chloride channel defect might impair the MPO-hydrogen peroxide-chloride microbicidal function. Here, we compared the competence of killing PsA by neutrophils from normal donors and CF patients. The data demonstrate that the killing rate by CF neutrophils was significantly lower than that by normal neutrophils. CF neutrophils in a chloride-deficient environment had only one-third of the bactericidal capacity of normal neutrophils in a physiological chloride environment. These results suggest that CFTR-dependent chloride anion transport contributes significantly to killing PsA by normal neutrophils and when defective as in CF, may compromise the ability to clear PsA.
Figures



Similar articles
-
Neutrophil bactericidal activity and host defenses in cystic fibrosis: a narrative review.J Thorac Dis. 2023 Oct 31;15(10):5773-5783. doi: 10.21037/jtd-23-846. Epub 2023 Sep 22. J Thorac Dis. 2023. PMID: 37969285 Free PMC article. Review.
-
CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis.Biochemistry. 2006 Aug 29;45(34):10260-9. doi: 10.1021/bi060490t. Biochemistry. 2006. PMID: 16922501 Free PMC article.
-
Exposure of Pseudomonas aeruginosa to bactericidal hypochlorous acid during neutrophil phagocytosis is compromised in cystic fibrosis.J Biol Chem. 2019 Sep 6;294(36):13502-13514. doi: 10.1074/jbc.RA119.009934. Epub 2019 Jul 24. J Biol Chem. 2019. PMID: 31341024 Free PMC article.
-
RNA interference against CFTR affects HL60-derived neutrophil microbicidal function.Free Radic Biol Med. 2010 Dec 15;49(12):1872-80. doi: 10.1016/j.freeradbiomed.2010.09.012. Epub 2010 Sep 24. Free Radic Biol Med. 2010. PMID: 20870018 Free PMC article.
-
Harnessing Neutrophil Survival Mechanisms during Chronic Infection by Pseudomonas aeruginosa: Novel Therapeutic Targets to Dampen Inflammation in Cystic Fibrosis.Front Cell Infect Microbiol. 2017 Jun 30;7:243. doi: 10.3389/fcimb.2017.00243. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713772 Free PMC article. Review.
Cited by
-
Neutrophil bactericidal activity and host defenses in cystic fibrosis: a narrative review.J Thorac Dis. 2023 Oct 31;15(10):5773-5783. doi: 10.21037/jtd-23-846. Epub 2023 Sep 22. J Thorac Dis. 2023. PMID: 37969285 Free PMC article. Review.
-
Association of Hyperchloremia With Hospital Mortality in Critically Ill Septic Patients.Crit Care Med. 2015 Sep;43(9):1938-44. doi: 10.1097/CCM.0000000000001161. Crit Care Med. 2015. PMID: 26154934 Free PMC article.
-
Response of Pseudomonas aeruginosa to the Innate Immune System-Derived Oxidants Hypochlorous Acid and Hypothiocyanous Acid.J Bacteriol. 2020 Dec 18;203(2):e00300-20. doi: 10.1128/JB.00300-20. Print 2020 Dec 18. J Bacteriol. 2020. PMID: 33106346 Free PMC article.
-
Neutrophil dysfunction in the pathogenesis of cystic fibrosis.Blood. 2022 Apr 28;139(17):2622-2631. doi: 10.1182/blood.2021014699. Blood. 2022. PMID: 35213685 Free PMC article. Review.
-
Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR.PLoS One. 2011;6(9):e23637. doi: 10.1371/journal.pone.0023637. Epub 2011 Sep 1. PLoS One. 2011. PMID: 21909403 Free PMC article.
References
-
- Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. - PubMed
-
- Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. - PubMed
-
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–17. - PubMed
-
- Sbarra AJ, Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959;234(6):1355–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

