Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex
- PMID: 18353946
- PMCID: PMC2395204
- DOI: 10.1128/JVI.00162-08
Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex
Abstract
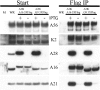
Deletion of the A56R or K2L gene of vaccinia virus (VACV) results in the spontaneous fusion of infected cells to form large multinucleated syncytia. A56 and K2 polypeptides bind to one another (A56/K2) and together are required for interaction with the VACV entry fusion complex (EFC); this association has been proposed to prevent the fusion of infected cells. At least eight viral polypeptides comprise the EFC, but no information has been available regarding their interactions either with each other or with A56/K2. Utilizing a panel of recombinant VACVs designed to repress expression of individual EFC subunits, we demonstrated that A56/K2 interacted with two polypeptides: A16 and G9. Both A16 and G9 were required for the efficient binding of each to A56/K2, suggesting that the two polypeptides interact with each other within the EFC. Such an interaction was established by the copurification of A16 and G9 from infected cells under conditions in which a stable EFC complex failed to assemble and from detergent-treated lysates of uninfected cells that coexpressed A16 and G9. A recombinant VACV that expressed G9 modified with an N-terminal epitope tag induced the formation of syncytia, suggesting partial interference with the functional interaction of A56/K2 with the EFC during infection. These data suggest that A16 and G9 are physically associated within the EFC and that their interaction with A56/K2 suppresses spontaneous syncytium formation and possibly "fuse-back" superinfection of cells.
Figures






References
-
- Armstrong, J. A., D. H. Metz, and M. R. Young. 1973. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 21533-537. - PubMed
-
- Brum, L. M., P. C. Turner, H. Devick, M. T. Baquero, and R. W. Moyer. 2003. Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology 306289-302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

